Unveiling genetic diversity and characterization of butterfly pea (Clitoria ternatea L.) germplasms using morphometric, biochemical, flow cytometry, and molecular markers
*Article not assigned to an issue yet
Bhusan Anwesa, Sarkar Jayoti Majumder, Subrahmanyeswari Tsama, Bandyopadhyay Sandipan, Chowdhuri Tapas Kumar, Gantait Saikat
Research Articles | Published: 23 June, 2025
First Page: 0
Last Page: 0
Views: 1000
Keywords: Antioxidants, Cyanidin, Flow cytometry, Micromorphology, Molecular markers
Abstract
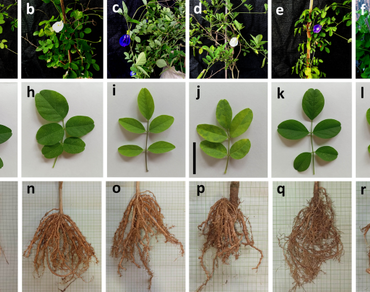
The present research focused on the identification and characterization of butterfly pea (Clitoria ternatea L.), employing a combination of morphological, micromorphological, biochemical, and molecular markers-based studies. Morphological characterization of six germplasms (Blue Single, White Single, Blue Double, White Double, Purple Single, and Purple Double) of butterfly pea revealed that two germplasms, namely, Blue Single and White Single, were similar in almost all morphological features but significantly different from the other four germplasms. Biochemical analyses on total phenol content and five antioxidant properties (DPPH, ABTS, FRAP assays, SOD, and POD activities) exhibited significant levels of variation across the germplasms. HPLC-based cyanidin quantification indicated substantial diversity among the germplasms, with the highest cyanidin content (773.43 mg/100 g fresh weight) was found in Purple Double. Flow cytometry-based comparison of ploidy levels revealed a double peak in the Purple Double, unlike a single peak in the other five germplasms, suggesting the possibility of mixoploidy in it. Additionally, molecular marker-based diversity was assessed using inter simple sequence repeats and start codon targeted polymorphism markers, resulting in 94 polymorphic bands, indicating 14.78% polymorphism. Purple Double was found to be completely distinct from the other germplasms and was placed separately in a different root cluster, making it a potential candidate for hybridization. This comprehensive analysis highlights the potential of butterfly pea for breeding programs, especially with the identified genetic diversity and distinct characteristics in the Purple Double germplasm.
References
Abidin ZHZ, Manah NSA, Hadi AN, Saugi NS, Fuad FA, Mazni NA, Hassan HC, Careem MA, Arof AK (2019) The colour stability of natural blue dye extracted from Clitoria ternatea L. in poly(acrylamide-co-acrylic acid) coating film. Pigment Resin Technol 48:265–271. https://doi.org/10.1108/prt-12-2017-0106
Ali Z, Ganie SH, Narula A, Sharma MP, Srivastava PS (2013) Intra-specific genetic diversity and chemical profiling of different accessions of Clitoria ternatea L. Ind Crops Prod 43:768–773. https://doi.org/10.1016/j.indcrop.2012.07.070
Bhattacharya P, Biswas S, Pal JK (2015) Palyno-taxonomic study of some plant taxa of Fabaceae from Arambagh region of Hooghly district, West bengal, Eastern India. Bioscience Discovery 6:27–34
Bishoyi AK, Geetha KA (2012) Polymorphism in flower colour and petal type in Aparajita (Clitoria ternatea). Open Access J Med Aromatic Plants 3:12–14. https://epubs.icar.org.in/index.php/JMAP/article/view/25996
Bishoyi AK, Pillai VV, Geetha KA, Maiti S (2014) Assessment of genetic diversity in Clitoria ternatea populations from different parts of India by RAPD and ISSR markers. Genet Resour Crop Evol 61:1597–1609. https://doi.org/10.1007/s10722-014-0145-y
Braca A, Tommasi ND, Di Bari L, Pizza C, Politi M, Morelli I (2001) Antioxidant principles from Bauhinia tarapotensis. J Nat Prod 64:892–895. https://doi.org/10.1021/np0100845
Chakraborthy GS, Kumar V, Gupta S, Kumar A, Gautam N, Kumari L (2018) Phytochemical and pharmacological aspects of Clitoria ternatea - a review. J Appl Pharm Sci Res 1:3–9. https://doi.org/10.31069/japsr.v1i2.13061
Chandra S, Das A, Roy T, Bose P, Mukherjee L, Samanta J, Banerjee R, Bakuli R, Jana M, Mukhopadhyay D (2019) Evaluation of methanolic extract of Clitoria ternatea hepatoprotective & nephroprotective activity in rats. J Drug Delivery Ther 9:313–319. https://doi.org/10.22270/jddt.v9i4-A.3478
Char M, Subrahmanyeswari T, Bhattacharyya S, Gantait S (2023) meta-Topolin-induced in vitro propagation, field evaluation, flow cytometry and molecular marker-based genetic stability assessment of potato cv. Badami Alu. Plant Cell Tissue Organ Cult 155:809–826. https://doi.org/10.1007/s11240-023-02601-8
Chen J, Li Z, Maiwulanjiang M, Zhang WL, Zhan JY, Lam CT, Zhu KY, Yao P, Choi RCY, Lau DTW, Dong TTX, Tsim KWK (2013) Chemical and biological assessment of Ziziphus jujuba fruits from china: different geographical sources and developmental stages. J Agric Food Chem 61:7315–7324. https://doi.org/10.1021/jf402379u
Chettri K, Majumder J, Mahanta M, Mitra M, Gantait S (2024) Genetic diversity analysis and molecular characterization of tropical Rose (Rosa spp.) varieties. Sci Hort 332:113243. https://doi.org/10.1016/j.scienta.2024.113243
Christodoulou MC, Orellana Palacios JC, Hesami G, Jafarzadeh S, Lorenzo JM, Domínguez R, Moreno A, Hadidi M (2022) Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 11:2213. https://doi.org/10.3390/antiox11112213
Cronk QC (2006) Legume flowers bear fruit. Proc Natl Acad Sci 103:4801–4802. https://doi.org/10.1073/pnas.0601298103
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42. https://doi.org/10.2307/3001478
Duy ND, Nguyen TMH, Lam TD, Pham TN, Thanh TT (2020) Extraction and determination of antioxidant activity of Vietnamese butterfly pea (Clitoria ternatea L). Mater Sci Forum 977:207–211. https://doi.org/10.4028/www.scientific.net/MSF.977.207
Facanali R, Colomboa CA, Teixeira JPF, Ming LC, Zucchi MI, Marquesa MOM (2015) Genetic and chemical diversity of native populations of Ocimum Selloi Benth. Ind Crops Prod 76:249–257. https://doi.org/10.1016/j.indcrop.2015.06.045
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051. https://doi.org/10.1126/science.220.4601.1049
Ganie SH, Ali Z, Das S, Srivastava PS, Sharma MP (2015) Genetic diversity and chemical profiling of different populations of Convolvulus Pluricaulis (Convolvulaceae): an important herb of ayurvedic medicine. 3 Biotech 5:295–302. https://doi.org/10.1007/s13205-014-0227-8
Geetha KA, Kawane A, Bishoyi AK, Phurailatpam A, Ankita C, Malik SK, Srinivasan R, Bhat SR (2013) Characterization of mode of reproduction of Commiphora Wwightii [(Arnott) bhandari] reveals novel pollen-pistil interaction and occurrence of obligate sexual female plants. Trees 27:567–581. https://doi.org/10.1007/s00468-012-0822-8
Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistical software package for education and data analysis. Palaeontologia Electronica 4:1–9
Handayani L, Aprilia S, Arahman N, Bilad MR (2024) Identification of the anthocyanin profile from butterfly pea (Clitoria ternatea L.) flowers under varying extraction conditions: evaluating its potential as a natural blue food colorant and its application as a colorimetric indicator. S Afr J Chem Eng 49:151–161. https://doi.org/10.1016/j.sajce.2024.04.008
Havananda T, Luengwilai K (2019) Variation in floral antioxidant activities and phytochemical properties among butterfly pea (Clitoria ternatea L.) germplasm. Genet Resour Crop Evol 66:645–658. https://doi.org/10.1007/s10722-018-00738-6
Khatoon S, Irshad S, Rawat AKS, Misra PK (2015) Comparative pharmacognostical studies of blue and white flower varieties of Clitoria ternatea L. J Pharmacognosy Nat Prod 1:1–6. https://doi.org/10.4172/2472-0992.1000109
Koley T, Majumder J, Mahanta M, Chowdhuri TK, Gantait S (2024) Characterization and diversity assessment of Hibiscus germplasms using morphological, biochemical and molecular markers. South Afr J Bot 169:164–177. https://doi.org/10.1016/j.sajb.2024.04.019
Kumari P, Raju DVS, Prasad KV, Saha S, Panwar S, Paul S, Banyal N, Bains A, Chawla P, Fogarasi M, Fogarasi S (2022) Characterization of anthocyanins and their antioxidant activities in Indian Rose varieties (Rosa × hybrida) using HPLC. Antioxidants 11:2032. https://doi.org/10.3390/antiox11102032
Lakshan SAT, Pathirana CK, Jayanath NY, Abeysekara WPKM, Abeysekara W, K.S.M (2020) Antioxidant and selected chemical properties of the flowers of three different varieties of butterfly pea (Clitoria Ternatea L). Ceylon J Sci 49:195–201. https://doi.org/10.4038/cjs.v49i2.7740
Lijon MB, Meghla NS, Jahedi E, Rahman MA, Hossain I (2017) Phytochemistry and Pharmacological activities of Clitoria ternatea. Int J Nat Social Sci 4:1–10
Ma YP, Wei JX, Yu ZY, Qin B, Dai SL (2015) Characterization of ploidy levels in Chrysanthemum L. by flow cytometry. J Forestry Res 26:771–775. https://doi.org/10.1007/s11676-015-0071-7
Mahmad N, Taha RM, Othman R, Elias H, Saleh A (2016) Encapsulated embryogenic callus of Clitoria ternatea l. for regeneration and conservation. Int J Environ Sci Dev 7:363–367
Makasana J, Dholakiya BZ, Gajbhiye NA, Bishoyi AK, Raju S (2016) Assessment of chemical diversity in Clitoria ternatea accessions by an improved and validated HPTLC method. Indian J Agric Sci 86:1133–1139. https://doi.org/10.56093/ijas.v86i9.61419
Miller NJ, Rice-Evans CA (1997) Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radic Res 26:195–199. https://doi.org/10.3109/10715769709097799
Morris JB (2009) Characterization of butterfly pea (Clitoria ternatea L.) accessions for morphology, phenology, reproduction and potential nutraceutical, pharmaceutical trait utilization. Genet Resour Crop Evol 56:421–427. https://doi.org/10.1007/s10722-008-9376-0
Mukherjee PK, Kumar V, Kumar NS, Heinrich M (2008) The ayurvedic medicine Clitoria ternatea—from traditional use to scientific assessment. J Ethnopharmacol 120:291–301. https://doi.org/10.1016/j.jep.2008.09.009
Naik A, Patel AK, Mishra SK, Nag A, Panigrahi J (2019) Characterization of intraspecific hybrid in Clitoria ternatea (L.) using morpho-physiological, cytogenetic, metabolic and molecular markers. Caryologia 72:11–22. https://doi.org/10.13128/caryologia-754
Nora S, Castro S, Loureiro J, Gonçalves AC, Oliveira H, Castro M, Santos C, Silveira P (2013) Flow cytometric and karyological analyses of Calendula species from Iberian Peninsula. Plant Syst Evol 299:853–864. https://doi.org/10.1007/s00606-013-0767-0
Nurhasanah, Hindersah R, Suganda T, Concibido V, Sundari, Karuniawan A (2023) The first report on the application of ISSR markers in genetic variance detection among butterfly pea (Clitoria ternatea L.) Accession in North Maluku Province, Indonesia. Horticulturae 9, 1059. https://doi.org/10.3390/horticulturae9091059
Oguis GK, Gilding EK, Jackson MA, Craik DJ (2019) Butterfly pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front Plant Sci 10:645. https://doi.org/10.3389/fpls.2019.00645
Olivas-Aguirre FJ, Rodrigo-García J, Martínez-Ruiz ND, Cárdenas-Robles AI, Mendoza-Díaz SO, Álvarez-Parrilla E, González-Aguilar GA, De la Rosa LA, Ramos-Jiménez A, Wall-Medrano A (2016) Cyanidin-3-O-glucoside: physical-chemistry, foodomics and health effects. Molecules 21:1264. https://doi.org/10.3390/molecules21091264
Petruk G, Del Giudice R, Rigano MM, Monti DM (2018) Antioxidants from plants protect against skin photoaging. Oxid Med Cell Longev, 2018, 1454936. https://doi.org/10.1155/2018/1454936
Pfenninger M, Véla E, Jesse R, Arantzazu Elejalde M, Liberto F, Magnin F, Martínez-Ortí A (2010) Temporal speciation pattern in the western Mediterranean genus Tudorella P. Fischer, 1885 (Gastropoda, Pomatiidae) supports the Tyrrhenian vicariance hypothesis. Mol Phylogenet Evol 54: 427–436. https://doi.org/10.1016/j.ympev.2009.09.024
Pullaiah T (2000) Embryology of Clitoria ternatea (Fabaceae). Plant Biosystems 134:39–43. https://doi.org/10.1080/11263500012331350325
Ravindran DR, Bharathithasan M, Ramaiah P, Mat Rasat MS, Rajendran D, Srikumar S, Ishak IH, Said AR, Ravi R, Mohd Amin MF (2020) Chemical composition and larvicidal activity of flower extracts from Clitoria ternatea against Aedes (Diptera: Culicidae). J Chem 2020:1–9. https://doi.org/10.1155/2020/3837207
Rewers M, Jedrzejczyk I (2016) Genetic characterization of Ocimum genus using flow cytometry and inter-simple sequence repeat markers. Ind Crops Prod 91:142–151. https://doi.org/10.1016/j.indcrop.2016.07.006
Rout K, Swain SS, Chand P (2014) Quantification of β-sitosterol in hairy root cultures and natural plant parts of butterfly pea (Clitoria ternatea L). J Planar Chromatogr 27:42–46. https://doi.org/10.1556/JPC.27.2014.1.8
Sahu D, Sahu JK, Kumar V, Tamrakar SK (2023) Phytochemicals and medicinal uses of Clitoria ternatea. Int J Plant Soil Sci 35:942–951. https://doi.org/10.9734/ijpss/2023/v35i183405
Shaaban A, Mahfouz H, Megawer EA, Saudy HS (2023) Physiological changes and nutritional value of forage clitoria grown in arid agro-ecosystem as influenced by plant density and water deficit. J Soil Sci Plant Nutr 23:3735–3750. https://doi.org/10.1007/s42729-023-01294-4
Shamnad J, Mathew D (2019) Karyomorphological studies on seven variants of Clitoria ternatea L. (Fabaceae). Trop Plant Res 6:320–325. https://doi.org/10.22271/tpr.2019.v6.i2.041
Solomon Raju AJ, Venkata Ramana K (2021) A study on pollination ecology of butterfly pea, Clitoria ternatea L. (Fabaceae). Species 22:29–35
Suarna IW, Wijaya IMS (2021) Butterfly pea (Clitoria ternatea L.: Fabaceae) and its morphological variations in Bali. J Trop Biodivers Biotechnol 6:1–12. https://doi.org/10.22146/jtbb.63013
Subrahmanyeswari T, Gantait S, Kamble SN, Singh S, Bhattacharyya S (2023) meta-Topolin-induced regeneration and ameliorated rebaudioside-A production in genetically uniform candy-leaf plantlets (Stevia rebaudiana Bert). South Afr J Bot 159:405–418. https://doi.org/10.1016/j.sajb.2023.05.045
Subrahmanyeswari T, Gantait S, Sarkar S, Bhattacharyya S (2022) Accelerated mono-phasic in vitro mass production of banana propagules and their morpho-cyto-genetic stability assessment. South Afr J Bot 146:794–806. https://doi.org/10.1016/j.sajb.2022.02.011
Surya D, Rajamani K, Suresh J, Uma D (2022) Morphological characterization and assessment of anthocyanin in three different genotypes of Clitoria ternatea L. Pharma Innov 11:2388–2392
Swathi KP, Jayaram S, Sugumar D, Rymbai E (2020) Evaluation of anti-inflammatory and anti-arthritic property of ethanolic extract of Clitoria ternatea. Chin Herb Med 13:243–249. https://doi.org/10.1016/j.chmed.2020.11.004
Thuy NM, Minh VQ, Ben TC, Nguyen MTT, Ha HTN, Tai NV (2021) Identification of anthocyanin compounds in butterfly pea flowers (Clitoria Ternatea L.) by ultra performance liquid chromatography/ultraviolet coupled to mass spectrometry. Molecules 26:4539. https://doi.org/10.3390/molecules26154539
Tuan Putra T, Zainol MK, Mohd Isa NS, MohdMaidin N (2021) Chemical characterization of ethanolic extract of butterfly pea flower (Clitoria ternatea). Food Res 5:127–134. https://doi.org/10.26656/fr.2017.5(4).744
Vidana Gamage GC, Lim YY, Choo WS (2021) Anthocyanins from Clitoria ternatea flower: biosynthesis, extraction, stability, antioxidant activity, and applications. Front Plant Sci 12:792303. https://doi.org/10.3389/fpls.2021.792303
Wang Z, Luo Y, Li X, Wang L, Xu S, Yang J, Weng L, Sato S, Tabata S, Ambrose M, Rameau C, Feng X, Hu X, Luo D (2008) Genetic control of floral zygomorphy in pea (Pisum sativum L). Proc Natl Acad Sci 105:10414–10419. https://doi.org/10.1073/pnas.0803291105
Yeotkar SD, Malode SN, Waghmare VN, Thakre P (2011) Genetic relationship and diversity analysis of Clitoria ternatea variants and Clitoria biflora using random amplified polymorphic DNA (RAPD) markers. Afr J Biotechnol 10:18065–18070. https://doi.org/10.5897/AJB11.1423
Yoshida K, Mori M, Kondo T (2009) Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat Prod Rep 26:884–915. https://doi.org/10.1039/b800165k
Zagórska-Dziok M, Ziemlewska A, Bujak T, Nizioł-Łukaszewska Z, Hordyjewicz-Baran Z (2021) Cosmetic and dermatological properties of selected ayurvedic plant extracts. Molecules 26:614. https://doi.org/10.3390/molecules26030614
Priya M., Balakrishnan V., Lakshmi A.K., Ramachandran A., Ravindran K.C. (2014). Mercury induced oxidative stress of antioxidants in Clitoria ternatea L. Int Lett Nat Sci 23: 1–8. https://doi.org/10.18052/www.scipress.com/ILNS.23.1
Author Information
Department of Floriculture and Landscape Architecture, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, Nadia, India