In vitro biological effects and in silico docking properties of different fractions obtained from Gymnocarpos decander Forsk aerial part collected in the wilaya of Naama
*Article not assigned to an issue yet
Aissaoui Mohammed, Sarra Chachoua, Manal Bendjerad, Wafa Nouari, Nadia Aissaoui
Research Articles | Published: 25 December, 2025
First Page: 0
Last Page: 0
Views: 589
Keywords: n Gymnocarpos decander forsk aerial part, Antioxidant activity, Antimicrobial activity, Hemolytic effect
Abstract
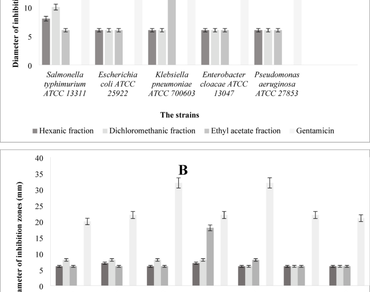
The objective of this study is to ascertain the biological potencies of the various fractions obtained from the aerial part of Gymnocarpos decander Forsk (El djefna). The estimation of polyphenols and flavonoids was conducted. The antimicrobial effect was carried out in accordance with the CLSI recommendations, employing a range of reference microorganisms. Meanwhile, the antioxidant potency was determined using means of the DPPH, ABTS and FRAP techniques. The hemolytic activity was determined, and the interaction between bioactive substances with SAP2 and SAP3 was investigated using molecular docking. The results indicate that the ethyl acetate fraction contains high levels of polyphenols and flavonoids (17.587 µEAG/mg MS and 25.456 µEC/mg MS respectively). The antimicrobial effects indicate that the hexanic fraction has the potential activity against L. monocytogenes ATCC 15,313 and Bacillus subtilis ATCC 6633 with a concentration of 2.5 mg/mL. However, it has been demonstrated that the ethyl acetate fraction is particularly effective against Candida albicans IP444 with a MIC of 5 mg/mL. The antioxidant activity shows that ethyl acetate and hexanic fractions have better effects in which the ABTS, FRAP and DPPH assays were values ranged between 3.22 µg/mL and 7.82 µg/mL. The hemolytic test revealed that none of the fractions are toxic, and the molecular docking suggested that El djefna could be useful in developing new drugs.
References
Abouri M, El Mousadik A, Msanda F, Boubaker H, Saadi B, Cherifi K (2012) An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int J Med Plants Res 1:99–123
Aissaoui M, Rahmoun NM, Barek S, Bensouici C, Abdelhamid El Haci I (2020) Structural characterization of phenolic content, antioxidant and antibacterial activities of Coffea Arabica green seeds. Vegetos 33:466–474
Amin K, Dannenfelser RM (2006) In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharm Sci 95:1173–1176
Bechlem H, Mencherini T, Bouheroum M, Benayache S, Cotugno R, Braca A, De Tommasi N (2017) New constituents from Gymnocarpos decander. Planta Med 83:1200–1206
Betts JW, Kelly SM, Haswell SJ (2011) Antibacterial effects of Theaflavin and synergy with epicatechin against clinical isolates of Acinetobacter baumannii and Stenotrophomonas maltophilia. Int J Antimicrob Agents 38:421–425
Biyiti LF, Meko’o DJL, Tamzc V, AmvamZollo PH (2004) Recherche de l’activité antibactérienne de quatre plantes médicinales camerounaises. Pharma Méd Trad Afr 13:11–20
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1119–1200
Borelli C, Ruge E, Schaller M, Monod M, Korting HC, Huber R, Maskos K (2007) The crystal structure of the secreted aspartic proteinase 3 from Candida albicans and its complex with pepstatin A. Proteins 68:738–748
Bouarab-Chibane L, Forquet V, Lantéri P, Clément Y, Léonard-Akkari L, Oulahal N, Degraève P, Bordes C (2019) Antibacterial properties of polyphenols: characterization and QSAR (Quantitative structure-activity relationship) models. Front Microbiol 10:829
Bouaziz M, Dhouib A, Loukil S, Boukhris M, Sayadi S (2009) Polyphenols content, antioxidant and antimicrobial activities of extracts of some wild plants collected from the South of Tunisia. Afr J Biotechnol 8:7017–7027
Cazarolli LH, Zanatta L, Alberton EH, Bonorino Figueiredo MSR, Folador P, Damazio RG, Pizzolatti MG, Barreto Silva FRM (2008) Flavonoids: prospective drug candidates. Mini Rev Med Chem 8:1429–1440
Clinical Laboratory Standards Institute-CLSI (2009) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard. CLSI document. M07-A8. Clinical and Laboratory Standards Institute, Wayane
Colina AR, Aumont F, Deslauriers N, Belhumeur P, de Repentigny L (1996) Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Infect Immun 64:4514–4519
Cutfield S, Dodson E, Anderson B, Moody P, Marshall C, Sullivan P, Cutfield J (1995) The crystal structure of a major secreted aspartic proteinase from Candida albicans in complexes with two inhibitors. Structure 3:1261–1271
Dimić D, Milenković D, Markovic Z, Marković JD (2019) The reactivity of dopamine precursors and metabolites towards ABTS•-: an experimental and theoretical study. J Serb Chem Soc 84:877–889
Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem 97:654–660
Farhadi F, Khameneh B, Iranshahi M, Iranshahy M (2019) Antibacterial activity of flavonoids and their structure-activity relationship: an update review. Phytother Res 33:13–40
Fathy H (2021) Polyphenolics from Gymnocarpos decandrus Forssk roots and their biological activities. Nat Prod Res 35:858–862
Gholam GM, Firdausy IA, Artika IM, Abdillah RM, Firmansyah RP (2023) Molecular docking: bioactive compounds of mimosa pudica as an inhibitor of Candida albicans Sap 3. Curr Biochem 10:24–37
Górniak I, Bartoszewski R, Króliczewski J (2019) Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 18:241–272
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17
Kozłowska J, Grela E, Baczyńska D, Grabowiecka A, Anioł M (2019) Novel O-alkyl derivatives of naringenin and their oximes with antimicrobial and anticancer activity. Molecules 24:679
Kumaran A, Karunakaran RJ (2007) In vitro antioxidant activities of methanol extracts of five phyllanthus species from India. LWT Food Sci Technol 40:344–352
Kurek A, Grudniak AM, Kraczkiewicz-Dowjat A, Wolska KI (2011) New antibacterial therapeutics and strategies. Pol J Microbiol 60:3–12
Kwun MS, Lee DG (2020) Quercetin-induced yeast apoptosis through mitochondrial dysfunction under the accumulation of magnesium in Candida albicans. Fungal Biol 124:83–90
Lee JY, Cho PY, Kim TY, Kang SY, Song KY, Hong SJ (2002) Hemolytic activity and developmental expression of pore-forming peptide, clonorin. Biochem Biophys Res Commun 296:1238–1244
Leopoldini M, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125:288–306
Naglik JR, Challacombe SJ, Hube B (2003) Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev 67:400–428
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open babel: an open chemical toolbox. J Cheminform 3:33
Omidfar F, Gheybi F, Zareian M, Karimi E (2023) Polyphenols in food industry, nano-based technology development and biological properties: an overview. eFood 4:e88
Ouedraogo S, Yoda J, Traore TK, Nitiema M, Sombie BC, Diawara HZ, Yameogo JB, Djande A, Belemnaba L, Kini FB, Ouedraogo S (2021) Production de matières premières et fabrication des médicaments à base de plantes médicinales. IJBCSC 15:750–772
Oyaizu M (1986) Study on products of browing reactions: antioxidative activities of browing reaction prepared from glucosamine. JPN J Nutr 44:307–315
Pengfei L, Tiansheng D, Xianglin H, Jianguo W (2009) Antioxidant properties of isolated isorhamnetin from the sea Buckthorn Marc. Plant Foods Hum Nutr 64:141–145
Perron NR, Brumaghim JL (2009) A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys 53:75–100
Qi W, Qi W, Xiong D, Long M (2022) Quercetin: its antioxidant Mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules 27:6545
Rashmi R, Magesh SB, Ramkumar KM, Suryanarayanan S, Subbarao MV (2018) Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep Biochem Mol Biol 7:76–84
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 26:1231–1237
Richardson JP, Ho J, Naglik JR (2018) Candida–Epithelial interactions. J Fungi 4:22
Sadeghi Z, Valizadeh J, Shermeh OA, Akaberi M (2015) Antioxidant activity and total phenolic content of Boerhavia elegans (choisy) grown in Baluchestan, Iran. Avicenna J Phytomed 5:1–9
Saleem M, Nazir M, Ali MS, Hussain H, Lee YS, Riaz N, Jabbar A (2010) Antimicrobial natural products: an update on future antibiotic drug candidates. Nat Prod Rep 27:238–254
Sandhar HK, Kumar B, Prasher S, Tiwari P, Salhan M, Sharma PA (2011) Review of phytochemistry and pharmacology of flavonoids. IPS 1:25–41
Shah SA (2022) Antimicrobial resistance. JKCD 4:12
Sofowora A (2010) Medicinal plants and traditional medicine in Africa, Spectrum Books Limited, Ibadan, Nigeria, 1st ed. John Wiley & Sons Limited, 1982
Talapko J, Juzbašić M, Matijević T, Pustijanac E, Bekić S, Kotris I, Škrlec I (2021) Candida albicans-The virulence factors and clinical manifestations of infection. J Fungi 7:79
Tang K, Zhao H (2023) Quinolone antibiotics: resistance and therapy. Infect Drug Resist 10:811–820
Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, Biraghi E, Canton E, Zimmermann K, Seaton S, Grillot R (2004) ECMM working group on Candidaemia. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis 23:317–322
Traoré Y, Ouattara K, Yéo D, Doumbia I, Coulibaly A (2012) Recherche des activités antifongique et antibactérienne des feuilles d’Annonasenegalensis Pers.(Annonaceae). J Appl Biosci 58:4234–4242
Trott O, Olson AJ (2010) AutoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Vermerris W, Nicholson R (2006) Isolation and identification of phenolic compounds: a practical guide. In: Phenolic compound biochemistry. Springer, Dordrecht, pp 151–196
Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A (2007) Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun 75:2126–2135
Waheed Y (2024) Clinical aspects of infectious diseases. J Clin Med 13:4853
Xie Y, Hua H, Zhou P (2022) Magnolol as a potent antifungal agent inhibits Candida albicans virulence factors via the PKC and Cek1 MAPK signaling pathways. Front Cell Infect Microbiol 12:935322
Yadav RNS, Agarwala M (2011) Phytochemical analysis of some medicinal plants. J Phytol 3:10–14
Yazdanparast R, Ardestani A (2007) In vitro antioxidant and free radical scavenging activity of Cyperus rotundus. J Med Food 10:667–674
Zitouni A, BelyagoubI-Benhammou N, El Zerey-Belaskri A, Toul F, Ghembaza N, Atik-Bekkara F (2021) Polyphenolic profile and comparative study on phytochemicals and antioxidant activity of extracts from all parts of Gymnocarpos decander Forsk. JNPRA 1:31–44
Author Information
Laboratoire Antibiotiques Antifongiques: Physicochimie, Synthèse et Activité Biologique, Department of Biology, Université de Tlemcen. EX. Complexe Biomédicale, Tlemcen, Algeria