Different preparation and characterization techniques of lipid nanoparticle and its application as drug carrier
*Article not assigned to an issue yet
Kumari Kajal, Shrestha Ishita, Singh Hare Ram, Sampath Muthu Kumar
Review Articles | Published: 28 December, 2025
First Page: 0
Last Page: 0
Views: 695
Keywords: Solid lipid nanoparticles, Drug delivery systems, mRNA delivery, Preparation-characterization techniques
Abstract
Lipid nanoparticles have emerged as versatile and biocompatible nanocarriers, capable of improving the solubility, stability, and bioavailability of hydrophilic and hydrophobic therapeutic agents. This review summarizes major preparation techniques, which comprise high-pressure homogenization, solvent emulsification-evaporation, solvent injection, microemulsion templating, phase inversion temperature processing, and supercritical fluid-based methods, with the emphasis on their operational principles, advantages, and limitations. The most relevant characterization strategies, like particle sizing, surface charge analysis, crystallinity assessment, and microscopic imaging, are introduced with the aim of emphasizing their importance to determine critical physicochemical quality and performance attributes of LNPs. The review further examines the current applications of LNPs in drug delivery, including gene therapy, mRNA vaccines, monoclonal antibody expression, and regenerative medicine, pointing out their ability to protect labile molecules, enhance targeting, and provide for controlled release. Besides established capabilities, this manuscript discusses the major challenges that constrain broader clinical translation: namely, formulation instability, limited loading of certain drugs, processing variability during scale-up, and inconsistent in vivo behaviour driven by protein corona formation and rapid clearance. Future advances are foreseen with rational lipid design, hybrid lipid–polymer systems, continuous-flow manufacturing, and computational formulation tools, which together hold promise to address these bottlenecks and extend the therapeutic potential of LNPs. Overall, this review provides a consolidated perspective on current methodologies, functional attributes, emerging challenges, and forward-looking strategies that will shape the next generation of lipid nanoparticle-based drug delivery systems.
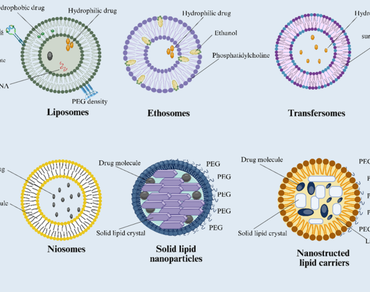
References
Ahmed TA (2015) Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J Liposome Res 25(1):1–10 https://doi.org/10.3109/08982104.2014.950276
Aji Alex MR, Chacko AJ, Jose S, Souto EB (2011) Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci 42(1–2):11–18
Andonova V, Peneva P (2018) Characterization methods for solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC). Curr Pharm Des 23(43):6630–6642
Andrade LN, Oliveira DML, Chaud Mv, Alves TF, R, Nery M, da Silva CF, Gonsalves JKC, Nunes RS, Corrêa CB, Amaral RG, Sanchez-Lopez E, Souto EB, Severino P (2019) Praziquantel-solid lipid nanoparticles produced by supercritical carbon dioxide extraction: physicochemical characterization, release profile, and cytotoxicity. Molecules 24(21), 3881
Battaglia L, Gallarate M, Cavalli R, Trotta M (2010) Solid lipid nanoparticles produced through a coacervation method. J Microencapsul 27(1):78–85
Bispo de Jesus M, Radaic A, Zuhorn IS, de Paula E (2013) Microemulsion extrusion technique: a new method to produce lipid nanoparticles. J Nanopart Res 15(10) 1960
Charcosset C, El-Harati A, Fessi H (2005) Preparation of solid lipid nanoparticles using a membrane contactor. J Control Release 108(1):112–120
Chen J, Wei N, Lopez-Garcia M, Ambrose D, Lee J, Annelin C et al (2017) Development and evaluation of resveratrol, vitamin E, and Epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur J Pharm Biopharm 117:286–291
Choi SH, Jin SE, Lee MK, Lim SJ, Park JS, Kim BG et al (2008) Novel cationic solid lipid nanoparticles enhanced p53 gene transfer to lung cancer cells. Eur J Pharm Biopharm 68(3):545–554
Chuang SY, Lin CH, Huang TH, Fang JY (2018) Lipid-Based nanoparticles as a potential delivery approach in the treatment of rheumatoid arthritis. Nanomaterials 8(1):1–16
Cölfen H, Antonietti M (2000) Field-flow fractionation techniques for polymer and colloid analysis. Adv Polym Sci 150:69–187
Dawoud M (2021) Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J Drug Deliv Sci Technol 62:102409
Ding Y, Nielsen KA, Nielsen BP, Bøje NW, Müller RH, Pyo SM (2018) Lipid-drug-conjugate (LDC) solid lipid nanoparticles (SLN) for the delivery of nicotine to the oral cavity – Optimization of nicotine loading efficiency. Eur J Pharm Biopharm 128:10–17
Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P et al (2020) A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv 10(45):26777–26791
Dubes A, Parrot-Lopez H, Abdelwahed W, Degobert G, Fessi H, Shahgaldian P et al (2003) Scanning electron microscopy and atomic force microscopy imaging of solid lipid nanoparticles derived from amphiphilic cyclodextrins. Eur J Pharm Biopharm 55(3):279–282
Duong VA, Nguyen TTL, Maeng HJ (2020) Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules 25(20):4781
Fadda P, Monduzzi M, Caboi F, Piras S, Lazzari P (2013) Solid lipid nanoparticle Preparation by a warm microemulsion based process: influence of microemulsion microstructure. Int J Pharm 446(1–2):166–175
Fang E, Liu X, Li M, Zhang Z, Song L, Zhu B et al (2022) Advances in COVID-19 mRNA vaccine development. Signal Transduct Target Ther 7(1)
Granja A, Lima-Sousa R, Alves CG, de Melo-Diogo D, Pinheiro M, Sousa CT et al (2021) Mitoxantrone-loaded lipid nanoparticles for breast cancer therapy – Quality-by-design approach and efficacy assessment in 2D and 3D in vitro cancer models. Int J Pharm 607:121044
Guada M, Beloqui A, Alhouayek M, Muccioli GG, del Carmen Dios-Viéitez M, Préat V et al (2016) Cyclosporine A-loaded lipid nanoparticles in inflammatory bowel disease. Int J Pharm 503(1–2):196–198
Gunduz U, Keskin T, Tansik G, Mutlu P, Yalcin S, Unsoy G et al (2014) Idarubicin-loaded folic acid conjugated magnetic nanoparticles as a targetable drug delivery system for breast cancer. Biomed Pharmacother 68(6):729–736
Hallan SS, Sguizzato M, Esposito E, Cortesi R (2021) Challenges in the physical characterization of lipid nanoparticles. Pharmaceutics 13(4):549. https://doi.org/10.3390/pharmaceutics13040549
Hecq J, Amighi K, Goole J (2016) Development and evaluation of insulin-loaded cationic solid lipid nanoparticles for oral delivery. J Drug Deliv Sci Technol 36:192–200
Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP (2002) A novel phase inversion-based process for the Preparation of lipid nanocarriers. Pharm Res 19(6):875–880
Ho HM, Huang CY, Cheng YJ, Shen KY, Tzeng TT, Liu SJ et al (2021) Assessment of adjuvantation strategy of lipid squalene nanoparticles for enhancing the immunogenicity of a SARS-CoV-2 Spike subunit protein against COVID-19. Int J Pharm 607:121024
Hou X, Zaks T, Langer R, Dong Y (2022) Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6(12):1078-94.
Iqbal M, Zafar N, Fessi H, Elaissari A (2015) Double emulsion solvent evaporation techniques used for drug encapsulation. Int J Pharm 496(2):173–190
Islan GA, Tornello PC, Abraham GA, Duran N, Castro GR (2016) Smart lipid nanoparticles containing Levofloxacin and DNase for lung delivery. Design and characterization. Colloids Surf B Biointerfaces 143:168–176
Jenning V, Mäder K, Gohla SH (2000) Solid lipid nanoparticles (SLN™) based on binary mixtures of liquid and solid lipids: a 1H-NMR study. Int J Pharm 205(1–2):15–21
Karunanidhi P, Ekambaram P, Abdul A, Sathali H, Priyanka K (2012) Solid lipid nanoparticles: A review. Sci Revs Chem Commun 2(1):80–102
Kaur IP, Bhandari R, Bhandari S, Kakkar V (2008) Potential of solid lipid nanoparticles in brain targeting. J Controll Release 127(2):97–109
Kulkarni JA, Cullis PR, van der Meel R (2018) Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther 28(3):146–157
Kumar R, Singh A, Garg N (2019) Acoustic cavitation assisted hot melt mixing technique for solid lipid nanoparticles formulation, characterization, and controlled delivery of poorly water-soluble drugs. J Drug Deliv Sci Technol 54:101277
Kumari K, Singh HR, Sampath MK (2024) Enhanced amoxicillin delivery via artificial intelligence (AI)-based optimized lipid nanoparticles for Helicobacter pylori. Biologia 80(1):133–148. https://doi.org/10.1007/s11756-024-01825-z
Manea AM, Andronescu C, Meghea A (2014) Green tea extract loaded into solid lipid nanoparticles. UPB Sci Bull Ser B: Chem Mater Sci 76(2):125–136
Martins SM, Sarmento B, Nunes C, Lúcio M, Reis S, Ferreira DC (2013) Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur J Pharm Biopharm 85(3):488–502
Mehnert W, Mäder K (2012) Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev 64(SUPPL):83–101
Monteiro N, Martins A, Reis RL, Neves NM (2015) Nanoparticle-based bioactive agent release systems for bone and cartilage tissue engineering. Regen Ther 1:109–118
Müller RH, Mäder K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the Art. Eur J Pharm Biopharm 50(1):161–177
Naseri N, Valizadeh H, Zakeri-Milani P (2015) Solid lipid nanoparticles and nanostructured lipid carriers: Structure, Preparation and application. Adv Pharm Bull 5(3):305–313
Notabi MK, Arnspang EC, Andersen M (2021) Antibody conjugated lipid nanoparticles as a targeted drug delivery system for hydrophobic pharmaceuticals. Eur J Pharm Sci 161:105777
Omwoyo WN, Ogutu B, Oloo F, Swai H, Kalombo L, Melariri P et al (2014) Preparation, characterization, and optimization of primaquine-loaded solid lipid nanoparticles. Int J Nanomed 9(1):3865–3874
Patel TK, Patel PB, Barvaliya M, Saurabh MK, Bhalla HL, Khosla PP (2021) Efficacy and safety of lopinavir-ritonavir in COVID-19: a systematic review of randomized controlled trials. J Infect Public Health 14(6):740–748
Pathak K (2021) Effective formulation strategies for poorly water soluble drugs. In: Advances and challenges in pharmaceutical technology: materials, process development and drug delivery strategies, pp 181–228
Pinheiro RGR, Granja A, Loureiro JA, Pereira MC, Pinheiro M, Neves AR et al (2020) Quercetin lipid nanoparticles functionalized with transferrin for alzheimer’s disease. Eur J Pharm Sci 148:105314
Piñón-Segundo E, Ganem-Quintanar A, Rafael Garibay-Bermúdez J, Juan Escobar-Chávez J, López-Cervantes M, Quintanar-Guerrero D (2006) Preparation of nanoparticles by solvent displacement using a novel recirculation system. Pharm Dev Technol 11(4):493–501
Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, et al (2009) Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carr Syst 26(6)
Rastogi SK, Zakrzewski M, Suryanarayanan R (2002) Investigation of solid-state reactions using variable temperature X-ray powder diffractometry. II. Aminophylline monohydrate. Pharm Res 19(9):1265–1273
Rawal SU, Patel MM (2018) Lipid nanoparticulate systems: modern versatile drug carriers. In: Lipid nanocarriers drug target, pp 49–138
Sakellari GI, Zafeiri I, Batchelor H, Spyropoulos F (2021) Formulation design, production and characterisation of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the encapsulation of a model hydrophobic active. Food Hydrocoll Health 1:100024
Salvioni L, Morelli L, Ochoa E, Labra M, Fiandra L, Palugan L et al (2021) The emerging role of nanotechnology in skincare. Adv Colloid Interface Sci 293:102437
Samimi S, Maghsoudnia N, Eftekhari RB, Dorkoosh F (2018) lipid-based nanoparticles for drug delivery systems. In: Characterization and biology of nanomaterials for drug delivery: nanoscience and nanotechnology in drug delivery, pp 47–76
Saupe A, Gordon KC, Rades T (2006) Structural investigations on nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers by cryo-field emission scanning electron microscopy and Raman spectroscopy. Int J Pharm 314(1):56–62
Schubert MA, Müller-Goymann CC (2003) Solvent injection as a new approach for manufacturing lipid nanoparticles – evaluation of the method and process parameters. Eur J Pharm Biopharm 55(1):125–131
Steiner D, Bunjes H (2021) Influence of process and formulation parameters on the Preparation of solid lipid nanoparticles by dual centrifugation. Int J Pharm X 3:100085
Sugimoto T (2019) Characterization of products. Monodispersed particles, pp 511 – 42
Suresh G, Manjunath K, Venkateswarlu V, Satyanarayana V (2007) Preparation, characterization, and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles. AAPS PharmSciTech 8(1)
Trotta M, Debernardi F, Caputo O (2003) Preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique. Int J Pharm 257(1–2)
Trucillo P, Campardelli R, Scognamiglio M, Reverchon E (2019) Control of liposomes diameter at micrometric and nanometric level using a supercritical assisted technique. J CO2 Util 32:119–127
Vinchhi P, Patel JK, Patel MM (2021) High-pressure homogenization techniques for nanoparticles. In: Emerging technologies for nanoparticle manufacturing, pp 263 – 85
Wang L, Wang CY, Zhang Y, Fu HJ, Gao Y, Zhang KR (2019) Preparation and characterization of solid lipid nanoparticles loaded with salmon calcitonin phospholipid complex. J Drug Deliv Sci Technol 52:838–845
Wang S, Chen T, Chen R, Hu Y, Chen M, Wang Y (2012) Emodin loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int J Pharm 430(1–2):238–246
Wang W, Chen K, Jiang T, Wu Y, Wu Z, Ying H, Yu H, Lu J, Lin J, Ouyang D (2024) Artificial intelligence-driven rational design of ionizable lipids for mRNA delivery. Nat Commun 15(1):10804. https://doi.org/10.1038/s41467-024-55072-6
Xu R (2008) Progress in nanoparticles characterization: sizing and zeta potential measurement. Particuology 6(2):112–115
Garcı́a-Fuentes M, Torres D, Alonso MJ (2003) Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules.Colloids Surf. B Biointerfaces 27(2–3):159–168. https://doi.org/10.1016/S0927-7765(02)00053-X
Khan MM, Madni A, Filipczak N, Pan J, Rehman M, Rai N, Attia SA, Torchilin VP (2020) Folate targeted lipid chitosan hybrid nanoparticles for enhanced anti-tumor efficacy. Nanomedicine: Nanotechnology, Biology and Medicine 28:102228. https://doi.org/10.1016/J.NANO.2020.102228
Ahmed MM, Fatima F, Anwer MK, Aldawsari MF, Alsaidan YSM, Alfaiz SA, Haque A, Az A, Alhazzani K (2020). Development and characterization of Brigatinib loaded solid lipid nanoparticles: In-vitro cytotoxicity against human carcinoma A549 lung cell lines. Chem Phys Lipids 233:105003. https://doi.org/10.1016/j.chemphyslip.2020.105003
Lee JM, Lim DS, Jeon SH, Hur D (2020) Zeta Potentials of Magnetite Particles and Alloy 690 Surfaces in Alkaline Solutions. Materials (Basel, Switzerland), 13. https://doi.org/10.3390/ma13183999
Ferreira M, Silva E, Barreiros L, Segundo MA, Costa Lima SA, Reis S (2016) Methotrexate loaded lipid nanoparticles for topical management of skin-related diseases: Design,characterization and skin permeation potential. Inter J Pharm 512(1):14–21. https://doi.org/10.1016/J.IJPHARM.2016.08.008
Author Information
Department of Bioengineering and Biotechnology, Birla Institute of Technology, Mesra, Ranchi, India