Phyto-chemical profiling, anti-oxidant and anti-microbial potential evaluation of the hydro-methanolic leaf extract of Aristolochia assamica, a new endemic species reported from N.E India
*Article not assigned to an issue yet
Dutta Aashis, Kakati Ranjit, Deka Nayanabhiram, Hussain Aziz, Ojah Raju, Borah Dipankar, Das Manas
Research Articles | Published: 17 July, 2025
First Page: 0
Last Page: 0
Views: 184
Keywords: n Aristolochia assamican , Anti-oxidant, Anti-microbial, FT-IR, LC-MS
Abstract
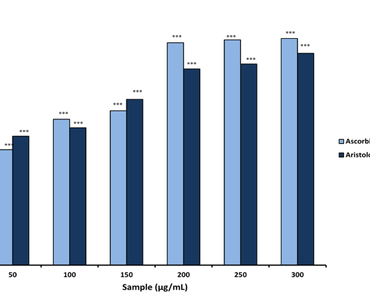
The exploration of medicinal plants for therapeutic application has been a long standing practice owing to their rich repository of bioactive compounds. This study aimed to analyze the chemical constituents along with anti-oxidant and anti-microbial potential evaluation of 70% hydro-methanolic leaf extract of Aristolochia assamica, an endemic medicinal plant of the East Himalayas (N.E India). Collection of Aristolochia assamica leaves was done from the fringe villages of Behali Reserve Forest present in Biswanath district of Assam. Following authentication, the 70% hydro-methanolic leaf extract (Net Yield = 103 mg/g dry weight) was subjected to anti-oxidant potential evaluation (DPPH assay, Total Anti-oxidant assay, NO assay and FRAP assay), anti-microbial assay, FT-IR profiling and LC-MS analysis. The results of DPPH radical scavenging assay and Total Anti-oxidant assay revealed the possession of moderately high DPPH radical scavenging property (IC50 = 58.63 ± 2.52 µg/mL; Standard (Ascorbic Acid) IC50 = 73.44 ± 0.22 µg/mL) and high total anti-oxidant potential with TEAC = 1.159 ± 0.014. Even the NO scavenging assay and FRAP assay demonstrate the moderate anti-oxidant potential of the extract (IC50 = 109.501 ± 1.033 µg/mL in case of NO assay and FRAP value of 9.98 ± 1.079 µg TE per mL of extract in case of FRAP assay). The anti-microbial assay revealed the possession of substantial anti-microbial activity against Pseudomonas aeruginosa, Kleibsella pneumoniae, Escherichia coli, Staphylococcus aureus and Enterococcus faecalis with ZOI = 17.67 mm, 15.33 mm, 19.33 mm, 20.33 mm and 23.33 mm respectively with MIC values within the range of 0.0125-0.05 mg/mL. The FT-IR profiling revealed the presence of functional groups like -CH2, =C-H, C-N, C-O, C = C, C = N, C = O, C-H, O-H and N-H, the outcome of which is substantiated by LC-MS analysis as it predicts the presence of a total of 16 phyto-constituents like 1-isothiocyanato-4-(methylsulfinyl)-butane, sennoside, papaverine, solasodine, kaempferol-3-O-rutinoside, codeinone, apigenin 7-O-rutinoside which possess those FT-IR predicted functional groups. Henceforth the hydro-methanolic leaf extract possesses high anti-oxidant and anti-microbial activity in turn providing substantial evidence for being a potentially rich source of anti-oxidants and anti-microbials.
References
Abdel-Haq H, Cometa MF, Palmery M, Leone MG, Silvestrini B, Saso L (2000) Relaxant effects of Hydrastis canadensis L. and its major alkaloids on guinea pig isolated trachea. Pharmacol Toxicol 87:218–222. https://doi.org/10.1034/j.1600-0773.2000.d01-77.x
Ahmad Rather M, Justin Thenmozhi A, Manivasagam T, Dhivya Bharathi M, Essa MM, Guillemin GJ (2018) Neuroprotective role of Asiatic acid in aluminium chloride induced rat model of alzheimer’s disease. Front Biosci (Schol Ed) 10:262–275. https://doi.org/10.2741/s514
Arora P, Nainwal LM, Gupta G, Singh SK, Chellappan DK, Oliver BG, Dua K (2022) Orally administered solasodine, a steroidal glycoalkaloid, suppresses ovalbumin-induced exaggerated Th2-immune response in rat model of bronchial asthma. Chem Biol Interact 366:110138. https://doi.org/10.1016/j.cbi.2022.110138
Arora R, Sawney S, Saini V, Steffi C, Tiwari M, Saluja D (2016) Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to KEAP1. Mol Cancer 15. https://doi.org/10.1186/s12943-016-0550-2
Bauer V, Kadlec O (1970) Antinicotinic properties of Papaverine in guinea pig taenia coli. Experientia 26:1331–1332. https://doi.org/10.1007/BF02113012
Benej M, Hong X, Vibhute S, Scott S, Wu J, Graves E, Le QT, Koong AC, Giaccia AJ, Yu B et al (2018) Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc Natl Acad Sci USA 115:10756–10761. https://doi.org/10.1073/pnas.1808945115
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292. PMID: 8660627
Berkó S, Zsikó S, Deák G, Gácsi A, Kovács A, Budai-Szűcs M, Pajor L, Bajory Z, Csányi E (2018) Papaverine hydrochloride containing nanostructured lyotropic liquid crystal formulation as a potential drug delivery system for the treatment of erectile dysfunction. Drug Des Devel Ther 12:2923–2931. https://doi.org/10.2147/DDDT.S168218
Bhaaskaran CT, Tamilselvi S, Bharathajothi P (2014) Antibacterial activity of Aristolochia bracteolata lam. Int J Inn Res Dev 3:319–321
Bisoli E, Garcez WS, Hamerski L, Tieppo C, Garcez FR (2008) Bioactive pentacyclic triterpenes from the stems of Combretum laxum. Molecules 13:2717–2728. https://doi.org/10.3390/molecules13112717
Borah D, Taram M, Das AP, Tangjang S, Do TV (2019) Aristolochia Assamica (Aristolochiaceae), a new species from the East Himalayas. Ann Bot Fenn 56:253–257
Borah PJ, Borah D, Das U, Das TJ, Sarma R (2021) A review on ethnopharmacological utility, traditional knowledge and phytochemistry of Aristolochia species in assam, India. Not Sci Biol 13:11027. https://doi.org/10.15835/nsb13311027
Bourhia M, Laasri FE, Moussa SI, Ullah R, Bari A, Saeed Ali S, Kaoutar A, Haj Said AA, El Mzibri M et al (2019) Phytochemistry, antioxidant activity, antiproliferative effect, and acute toxicity testing of two Moroccan Aristolochia species. Evid Based Complement Alternat Med 2019:9710876. https://doi.org/10.1155/2019/9710876
Campas-Baypoli ON, Bueno-Solano C, Martínez-Ibarra DM, Camacho-Gil F, Villa-Lerma AG, Rodríguez-Núñez JR, Lóez-Cervantes J, Sánchez-Machado DI (2009) Contenido, de Sulforafano (1-isotiocianato-4-(metilsulfinil)-butano) En vegetales crucíferos. Arch Latinoam Nutr 59:95–100
Chauhan K, Sheth N, Ranpariya V, Parmar S (2011) Anticonvulsant activity of Solasodine isolated from Solanum sisymbriifolium fruits in rodents. Pharm Biol 49:194–199. https://doi.org/10.3109/13880209.2010.508499
Chiba K, Yamazaki M, Kikuchi M, Kakuda R, Kikuchi M (2011) New physiological function of secoiridoids: neuritogenic activity in PC12h cells. J Nat Med 65:186–190. https://doi.org/10.1007/s11418-010-0449-y
Choi RY, Ham JR, Lee MK (2016) Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem Biol Interact 260:13–21. https://doi.org/10.1016/j.cbi.2016.10.013
Deng YT, Zhao MG, Xu TJ, Jin-Hou, Li XH (2018) Gentiopicroside abrogates lipopolysaccharide-induced depressive-like behavior in mice through tryptophan-degrading pathway. Metab Brain Dis 33:1413–1420. https://doi.org/10.1007/s11011-018-0246-y
Desai AB, Kagathara VG, Joshi H, Rangani AT, Mungra H (2011) Evaluation of antiamnesic effect of Solasodine in mice. Int J Pharm Tech Res 3:732–740
Dong SH, Liu YW, Wei F, Tan HZ, Han ZD (2017) Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin (BLM) via suppressing pro-fibrotic and inflammatory signaling pathways. Biomed Pharmacother 89:1297–1309. https://doi.org/10.1016/j.biopha.2017.03.005
Duncan SH, Leitch EC, Stanley KN, Richardson AJ, Laven RA, Flint HJ, Stewart CS (2004) Effects of Esculin and Esculetin on the survival of Escherichia coli O157 in human faecal slurries, continuous-flow simulations of the rumen and colon and in calves. Br J Nutr 91:749–755. https://doi.org/10.1079/BJN20041101
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR (2001) The anti-malarial Artesunate is also active against cancer. Int J Oncol 18:767–773. https://doi.org/10.3892/ijo.18.4.767
El Omari N, Sayah K, Fettach S, El Blidi O, Bouyahya A, Faouzi MEA, Kamal R, Barkiyou M (2019) Evaluation of in vitro antioxidant and antidiabetic activities of Aristolochia longa extracts. Evid Based Complement Alternat Med 2019:7384735. https://doi.org/10.1155/2019/7384735
Garratt DC (1964) The quantitative analysis of drugs. Chapman and Hall ltd, Japan
Goji H, Nadro M (2021) Phytochemical and In-vitro antioxidants potential of Aristolochia bracteolata root extract. J Complement Alternat Med 13:1–7. https://doi.org/10.9734/jocamr/2021/v13i330224
Gomes DA, Joubert AM, Visagie MH (2022) The biological relevance of Papaverine in Cancer cells. Cells 11:3385. https://doi.org/10.3390/cells11213385
Gulluce M, Orhan F, Adiguzel A, Bal T, Guvenalp Z, Dermirezer LO (2013) Determination of antimutagenic properties of apigenin-7-O-rutinoside, a flavonoid isolated from Mentha longifolia (L.) huds. Ssp. longifolia with yeast DEL assay. Toxicol Ind Health 29:534–540. https://doi.org/10.1177/0748233712442732
Guo B, Li X, Song S, Chen M, Cheng M, Zhao D, Li F (2016) (-)-β-hydrastine suppresses the proliferation and invasion of human lung adenocarcinoma cells by inhibiting PAK4 kinase activity. Oncol Rep 35:2246–2256. https://doi.org/10.3892/or.2016.4594
Gupta M, Mazumdar UK, Sivahkumar T, Vamis M, Karki SS, Ramanathan S et al (2003) Antioxidant and antiinflammatory activities of Acalypha fruticosa. Nig J Nat Prod Med 7. https://doi.org/10.4314/njnpm.v7i1.11700
Gupta RS, Dixit VP (2002) Effects of short-term treatment of Solasodine on cauda epididymis in dogs. Indian J Exp Biol 40:169–173
Hazra B, Biswas S, Mandal N (2008) Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med 8:63. https://doi.org/10.1186/1472-6882-8-63
Herath WH, Ferreira D, Khan IA (2003) Microbial transformation of the phthalideisoquinoline alkaloid, (-)-beta-hydrastine. Nat Prod Res 17:269–274. https://doi.org/10.1080/1057563021000060158
Hitosugi N, Hatsukari I, Ohno R, Hashimoto K, Mihara S, Mizukami S, Nakamura S, Sakagami H, Nagasaka H, Matsumoto I, Kawase M (2003) Comparative analysis of apoptosis-inducing activity of Codeine and codeinone. Anesthesiology 98:643–650. https://doi.org/10.1097/00000542-200303000-00012
Hong L, Xu Y, Wang D, Zhang Q, Li X, Xie C, Wu J, Zhong C, Fu J, Geng S (2023) Sulforaphane ameliorates bisphenol A-induced hepatic lipid accumulation by inhibiting Endoplasmic reticulum stress. Sci Rep 13:1147. https://doi.org/10.1038/s41598-023-28395-5
Hou TT, Yang HY, Wang W, Wu QQ, Tian YR, Jia JP (2018) Sulforaphane inhibits the generation of Amyloid-β oligomer and promotes Spatial learning and memory in alzheimer’s disease (PS1V97L) Transgenic mice. J Alzheimers Dis 62:1803–1813. https://doi.org/10.3233/JAD-171110
Hua F, Li JY, Zhang M, Zhou P, Wang L, Ling TJ, Bao GH (2022) Kaempferol-3-O-rutinoside exerts cardioprotective effects through NF-κB/NLRP3/Caspase-1 pathway in ventricular remodeling after acute myocardial infarction. J Food Biochem 46. https://doi.org/10.1111/jfbc.14305
Hurtado O, Ballesteros I, Cuartero MI, Moraga A, Pradillo JM, Ramírez-Franco J, Bartolomé-Martín D, Pascual D, Torres M, Sánchez-Prieto J et al (2012) Daidzein has neuroprotective effects through ligand-binding-independent PPARγ activation. Neurochem Int 61:119–127. https://doi.org/10.1016/j.neuint.2012.04.007
Jeong NH, Yang EJ, Jin M, Lee JY, Choi YA, Park PH, Lee SR, Kim SU, Shin TY, Kwon TK al (2018) Esculetin from Fraxinus rhynchophylla attenuates atopic skin inflammation by inhibiting the expression of inflammatory cytokines. Int Immunopharmacol 59:209–216. https://doi.org/10.1016/j.intimp.2018.04.005
Jiang H, Zhong J, Li W, Dong J, Xian CJ, Shen YK, Yao L, Wu Q, Wang L (2021) Gentiopicroside promotes the osteogenesis of bone mesenchymal stem cells by modulation of β-catenin-BMP2 signalling pathway. J Cell Mol Med 25:10825–10836. https://doi.org/10.1111/jcmm.16410
Kadlec O, Bauer V, Seferna I(1973) An indirect action of Papaverine in smooth muscle in vivo. Pharmacology 9:281–284. https://doi.org/10.1159/000136397
Kampkötter A, Chovolou Y, Kulawik A, Röhrdanz E, Weber N, Proksch P, Wätjen W (2008) Isoflavone Daidzein possesses no antioxidant activities in cell-free assays but induces the antioxidant enzyme catalase. Nutr Res 28:620–628. https://doi.org/10.1016/j.nutres.2008.06.002
Kavitha CV, Jain AK, Agarwal C, Pierce A, Keating A, Huber KM, Serkova NJ, Wempe MF, Agarwal R, Deep G (2015) Asiatic acid induces Endoplasmic reticulum stress and apoptotic death in glioblastoma multiforme cells both in vitro and in vivo. Mol Carcinog 54:1417–1429. https://doi.org/10.1002/mc.22220
Ke M, Hu XQ, Ouyang J, Dai B, Xu Y (2012) The effect of Astragalin on the VEGF production of cultured Müller cells under high glucose conditions. Biomed Mater Eng 22:113–119. https://doi.org/10.3233/BME-2012-0696
Khan AQ, Khan R, Rehman MU, Lateef A, Tahir M, Ali F, Sultana S (2012) Soy isoflavones (daidzein & genistein) inhibit 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced cutaneous inflammation via modulation of COX-2 and NF-κB in Swiss albino mice. Toxicology 302:266–274. https://doi.org/10.1016/j.tox.2012.08.008
Khan MA (2016) Introduction and importance of medicinal plants and herbs. Unani Zahid, India
Khaodhiar L, Ricciotti HA, Li L, Pan W, Schickel M, Zhou J, Blackburn GL (2008) Daidzein-rich isoflavone aglycones are potentially effective in reducing hot flashes in menopausal women. Menopause 15:125–132
Kim AJ, Park JE, Cho YH, Lim DS, Lee JS (2021) Effect of 7-Methylsulfinylheptyl isothiocyanate on the Inhibition of melanogenesis in B16-F1 cells. Life (Basel) 11:162. https://doi.org/10.3390/life11020162
Kim DH, Jung HA, Park SJ, Kim JM, Lee S, Choi JS, Cheong JH, Ko KH, Ryu JH (2010) The effects of Daidzin and its aglycon, daidzein, on the scopolamine-induced memory impairment in male mice. Arch Pharm Res 33:1685–1690. https://doi.org/10.1007/s12272-010-1019-2
Kim JW, Jin YC, Kim YM, Rhie S, Kim HJ, Seo HG, Lee JH, Ha YL, Chang KC (2009) Daidzein administration in vivo reduces myocardial injury in a rat ischemia/reperfusion model by inhibiting NF-kappaB activation. Life Sci 84:227–234. https://doi.org/10.1016/j.lfs.2008.12.005
Kim SH, Kang KA, Zhang R, Piao MJ, Ko DO, Wang ZH, Chae SW, Kang SS, Lee KH, Kang HK et al (2008) Protective effect of Esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol Sin 29:1319–1326. https://doi.org/10.1111/j.1745-7254.2008.00878.x
Klein-Júnior LC, Santin JR, Lemos M, Silveira AC, Rocha JA, Beber AP, Wagner TM, Bresolin TM, Bella-Cruz A, Cechinel-Filho V et al (2013) Role of gastric mucus secretion, oxinitrergic system and sulfhydryl groups on the gastroprotection elicited by Polygala cyparissias (Polygalaceae) in mice. J Pharm Pharmacol 65:767–776. https://doi.org/10.1111/jphp.12038
Kumarasamy Y, Nahar L, Sarker SD (2003) Bioactivity of Gentiopicroside from the aerial parts of Centaurium erythraea. Fitoterapia 74:151–154. https://doi.org/10.1016/s0367-326x(02)00319-2
Lal Shyaula S, Abbas G, Siddiqui H, Sattar SA, Choudhary MI, Basha FZ (2012) Synthesis and antiglycation activity of kaempferol-3-O-rutinoside (nicotiflorin). Med Chem 8:415–420. https://doi.org/10.2174/1573406411208030415
Lee SR, Lee S, Moon E, Park HJ, Park HB, Kim KH (2017) Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorg Chem 70:94–99. https://doi.org/10.1016/j.bioorg.2016.11.012
Li H, Shi R, Ding F, Wang H, Han W, Ma F, Hu M, Ma CW, Huang Z (2016) Astragalus polysaccharide suppresses 6-Hydroxydopamine-Induced neurotoxicity in Caenorhabditis elegans. Oxid Med Cell Longev 2016:4856761. https://doi.org/10.1155/2016/4856761
Li JF, Huang RZ, Yao GY, Ye MY, Wang HS, Pan YM, Xiao JT (2014) Synthesis and biological evaluation of novel aniline-derived Asiatic acid derivatives as potential anticancer agents. Eur J Med Chem 86:175–188. https://doi.org/10.1016/j.ejmech.2014.08.003
Li X, Yang C, Shen H (2019) Gentiopicroside exerts convincing antitumor effects in human ovarian carcinoma cells (SKOV3) by inducing cell cycle arrest, mitochondrial mediated apoptosis and Inhibition of cell migration. J BUON 24:280–284
Lim H, Kim HJ, Jeong H, Park HR (2017) Anti-inflammatory effects of 1-isothiocyanato-7-(methylsulfonyl) heptane by suppressing the NFκ-B signaling pathway. Eur J Inflamm 15:57–65. https://doi.org/10.1177/1721727X17719600
Lin JY, Tournas JA, Burch JA, Monteiro-Riviere NA, Zielinski J (2008) Topical isoflavones provide effective photoprotection to skin. Photodermatol Photoimmunol Photomed 24:61–66. https://doi.org/10.1111/j.1600-0781.2008.00329.x
Liu J, Chen L, Lu H (2018) Asiatic acid enhances antioxidant and Anti-inflammatory activity to suppress isoproterenol induced cardiotoxicity. Int J Pharmacol 14:1038–1045
Liu Q, Cheng L, Matsuura A, Xiang L, Qi J (2020) Gentiopicroside, a secoiridoid glycoside from Gentiana rigescens franch, extends the lifespan of yeast via inducing mitophagy and antioxidative stress. Oxid Med Cell Longev 2020:9125752. https://doi.org/10.1155/2020/9125752
Loganathan R, Vasugi SR (2018) In vitro antioxidant activity of Brassica oleracea, Aristolochia bracteolata leaves extract. J Pharmacogn Phytochem 7:2080–2084
Lv J, Sharma A, Zhang T, Wu Y, Ding X (2018) Pharmacological review on Asiatic acid and its derivatives: A potential compound. SLAS Technol 23:111–127. https://doi.org/10.1177/2472630317751840
Ma Y, Liu Y, Sun A, Du Y, Ye M, Pu X, Qi X (2017) Intestinal absorption and neuroprotective effects of kaempferol-3-O-rutinoside. RSC Adv 7:31408–31416. https://doi.org/10.1039/C7RA05415G
Martin D, Song J, Mark C, Eyster K (2008) Understanding the cardiovascular actions of soy isoflavones: potential novel targets for antihypertensive drug development. Cardiovasc Hematol Disord Drug Targets 8:297–312. https://doi.org/10.2174/187152908786786214
Mavondo GA, Mkhwananzi BN, Mabandla MV (2016) Pre-infection administration of Asiatic acid retards parasitaemia induction in plasmodium Berghei murine malaria infected Sprague-Dawley rats. Malar J 15:226. https://doi.org/10.1186/s12936-016-1278-6
Meng X, Zhang A, Wang X, Sun H (2019) A kaempferol-3-O-β-d-glucoside, intervention effect of Astragalin on estradiol metabolism. Steroids 149:108413. https://doi.org/10.1016/j.steroids.2019.05.005
Meng XM, Zhang Y, Huang XR, Ren GL, Li J, Lan HY (2015) Treatment of renal fibrosis by rebalancing TGF-β/Smad signaling with the combination of Asiatic acid and naringenin. Oncotarget 6:36984–36997. https://doi.org/10.18632/oncotarget.6100
Mushtaq Z, Imran M, Hussain M, Saeed M, Imran A, Umar M, Abdelgawad MA, El-Ghorab AH, Ahmed A, Alsagaby SA et al (2023) Asiatic acid: a review on its polypharmacological properties and therapeutic potential against various maladies. Int J Food Prop 26:1244–1263. https://doi.org/10.1080/10942912.2023.2209702
Negi PS, Anandharamakrishnan C, Jayaprakasha GK (2003) Antibacterial activity of Aristolochia bracteata root extracts. J Med Food 6:401–403. https://doi.org/10.1089/109662003772519994
Ohkoshi E, Miyazaki H, Shindo K, Watanabe H, Yoshida A, Yajima H (2007) Constituents from the leaves of Nelumbo nucifera stimulate lipolysis in the white adipose tissue of mice. Planta Med 73:1255–1259. https://doi.org/10.1055/s-2007-990223
Osawa T (1994) Novel natural antioxidants for utilization in food and biological systems. Post harvest biochem. Plant Food Mater Trop 12:241–251
Pandurangan A, Khosa RL, Hemalatha S (2010) Antinociceptive activity of steroid alkaloids isolated from Solanum trilobatum Linn. J Asian Nat Prod Res 12:691–695. https://doi.org/10.1080/10286020.2010.497997
Pandurangan A, Khosa RL, Hemalatha S (2011) Anti-inflammatory activity of an alkaloid from Solanum trilobatum on acute and chronic inflammation models. Nat Prod Res 25:1132–1141. https://doi.org/10.1080/14786410903370783
Park JH, Seo YH, Jang JH, Jeong CH, Lee S, Park B (2017) Asiatic acid attenuates methamphetamine-induced neuroinflammation and neurotoxicity through blocking of NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway. J Neuroinflammation 14:240. https://doi.org/10.1186/s12974-017-1009-0
Phongpaichit S, Nikom J, Rungjindamai N, Sakayaroj J, Hutadilok-Towatana N, Rukachaisirikul V, Kirtikara K (2007) Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol Med Microbiol 51:517–525. https://doi.org/10.1111/j.1574-695X.2007.00331.x
Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL, Nishizawa S, Kasuya H, Wellman G, Keller E, Zauner A, Dorsch N et al (2009) Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res 31:151–158. https://doi.org/10.1179/174313209X393564
Poletto Bonetto JH, Luz de Castro A, Fernandes RO, Corssac GB, Cordero EA, Schenkel PC, Sander da Rosa Araujo A, Belló-Klein A (2022) Sulforaphane effects on cardiac function and Calcium-Handling-Related proteins in 2 experimental models of heart disease: Ischemia-Reperfusion and infarction. J Cardiovasc Pharmacol 79:325–334. https://doi.org/10.1097/FJC.0000000000001191
Prabakaran D, Ashokkumar N (2013) Protective effect of Esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie 95:366–373. https://doi.org/10.1016/j.biochi.2012.10.008
Qin Y, Shu F, Zeng Y, Meng X, Wang B, Diao L, Wang L, Wan J, Zhu J, Wang J et al (2014) Daidzein supplementation decreases serum triglyceride and uric acid concentrations in hypercholesterolemic adults with the effect on triglycerides being greater in those with the GA compared with the GG genotype of ESR-β RsaI. J Nutr 144:49–54. https://doi.org/10.3945/jn.113.182725
Qu D, Han J, Ren H, Yang W, Zhang X, Zheng Q, Wang D (2016) Cardioprotective effects of Astragalin against myocardial ischemia/reperfusion injury in isolated rat heart. Oxid Med Cell Longev 2016:8194690. https://doi.org/10.1155/2016/8194690
Ramachandran V, Saravanan R, Senthilraja P (2014) Antidiabetic and antihyperlipidemic activity of Asiatic acid in diabetic rats, role of HMG coa: in vivo and in Silico approaches. Phytomedicine 21:225–232. https://doi.org/10.1016/j.phymed.2013.08.027
Ramchander, Jalwal P, Middha A (2017) Recent advances on senna as a laxative: A comprehensive review. J Pharmacogn Phytochem 6:349–353
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization Asszy. Free Rad Biol Med 26:1231–1237
Roghani M, Vaez Mahdavi MR, Jalali-Nadoushan MR, Baluchnejadmojarad T, Naderi G, Roghani-Dehkordi F, Taghi Joghataei M, Kord M (2013) Chronic administration of daidzein, a soybean isoflavone, improves endothelial dysfunction and attenuates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res 27:112–117. https://doi.org/10.1002/ptr.4699
Rojas A, Bah M, Rojas JI, Gutiérrez DM (2000) Smooth muscle relaxing activity of Gentiopicroside isolated from Gentiana spathacea. Planta Med 66:765–767. https://doi.org/10.1055/s-2000-9774
Romero MR, Efferth T, Serrano MA, Castaño B, Macias RI, Briz O, Marin JJ (2005) Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an in vitro replicative system. Antiviral Res 68:75–83. https://doi.org/10.1016/j.antiviral.2005.07.005
Santi R, Contessa AR, Ferrari M (1963) Spasmolytic effect of the Papaverine and Inhibition of the oxidative phosphorylation. Biochem Biophys Res Commun 11:156–159. https://doi.org/10.1016/0006-291x(63)90084-6
Setchell KD, Brown NM, Lydeking-Olsen E (2002) The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 132:3577–3584. https://doi.org/10.1093/jn/132.12.3577
Siuciak JA, Strick CA (2006) Treating neuropsychiatric disorders with PDE10A inhibitors. Drug Discov Today Ther Strateg 3:527–532
So HC, Chau CK, Chiu WT, Ho KS, Lo CP, Yim SH, Sham PC (2017) Analysis of genome-wide association data highlights candidates for drug repositioning in psychiatry. Nat Neurosci 20:1342–1349. https://doi.org/10.1038/nn.4618
Soumyakrishnan S, Divya T, Kalayarasan S, Sriram N, Sudhandiran G (2014) Daidzein exhibits anti-fibrotic effect by reducing the expressions of proteinase activated receptor 2 and TGFβ1/smad mediated inflammation and apoptosis in Bleomycin-induced experimental pulmonary fibrosis. Biochimie 103:23–36. https://doi.org/10.1016/j.biochi.2014.04.005
Subramaniyan V, Saravanan R, Baskaran D, Ramalalingam S (2015) In vitro free radical scavenging and anticancer potential of aristolochia indica L. against MCF-7 cell line. Int J Pharm Pharm Sci 7:392–396
Suliman Mohamed M, Timan Idriss M, Khedr AI, Abd AlGadir H, Takeshita S, Shah MM, Ichinose Y, Maki T (2014) Activity of Aristolochia bracteolata against Moraxella catarrhalis. Int J Bacteriol. 2014:481686. https://doi.org/10.1155/2014/481686
Taiwo FO, Oyedeji O, Osundahunsi MT (2019) Antimicrobial and antioxidant properties of kaempferol-3-O-glucoside and 1-(4-Hydroxyphenyl)-3-phenylpropan-1-one isolated from the leaves of Annona muricata (Linn). Jour Pharm Res Inter 26:1–13. https://doi.org/10.9734/jpri/2019/v26i330138
Takeuchi R, Hoshijima H, Onuki N, Nagasaka H, Chowdhury SA, Kawase M, Sakagami H (2005) Effect of anticancer agents on codeinone-induced apoptosis in human cancer cell lines. Anticancer Res 25:4037–4041
Thakor FK, Wan KW, Welsby PJ, Welsby G (2017) Pharmacological effects of Asiatic acid in glioblastoma cells under hypoxia. Mol Cell Biochem 430:179–190. https://doi.org/10.1007/s11010-017-2965-5
Trejo HE, Urich D, Pezzulo AA, Caraballo JC, Gutiérrez J, Castro IJ, Centeno GR, Sánchez de León R (2007) Beneficial effects of hydrocortisone and Papaverine on a model of pulmonary embolism induced by autologous blood clots in isolated and perfused rabbit lungs. Respirology 12:799–806. https://doi.org/10.1111/j.1440-1843.2007.01177.x
Utzinger J, Xiao SH, Tanner M, Keiser J (2007) Artemisinins for schistosomiasis and beyond. Curr Opin Investig Drugs 8:105–116
Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D (2004) Soy isoflavones suppress invasiveness of breast cancer cells by the Inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol 25:1389–1395
Wang J, Xu C, Wong Y, Li Y, Liao F, Jiang T, Tu Y (2018a) Artemisinin, the magic drug discovered from traditional Chinese medicine. Engineering 5:32–39. https://doi.org/10.1016/j.eng.2018.11.011
Wang LD, Guo DA, Yuan L, He QH, Hu YQ, Tu PF, Zheng JH (2000) Antifungal effect of three natural products on the genetic substance of Saccharomyces cerevisiae GL7 and prototheca wickerhamii. Yao Xue Xue Bao 35:860–863
Wang Q, Zhou X, Yang L, Luo M, Han L, Lu Y, Shi Q, Wang Y, Liang Q (2019) Gentiopicroside (GENT) protects against sepsis induced by lipopolysaccharide (LPS) through the NF-κB signaling pathway. Ann Transl Med 7:731
Wang QH, Qin SW, Jiang JG (2022) Improvement effects of Esculetin on the formation and development of atherosclerosis. Biomed Pharmacother 150:113001. https://doi.org/10.1016/j.biopha.2022.113001
Wang Z, Li Q, Xiang M, Zhang F, Wei D, Wen Z, Zhou Y (2017) Astragaloside alleviates hepatic fibrosis function via PAR2 signaling pathway in diabetic rats. Cell Physiol Biochem 41:1156–1166. https://doi.org/10.1159/000464122
Wang ZH, Mong MC, Yang YC, Yin MC (2018b) Asiatic acid and maslinic acid attenuated Kainic acid-induced seizure through decreasing hippocampal inflammatory and oxidative stress. Epilepsy Res 139:28–34. https://doi.org/10.1016/j.eplepsyres.2017.11.003
Wei L, Chen Q, Guo A, Fan J, Wang R, Zhang H (2018) Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int Immunopharmacol 60:1–8. https://doi.org/10.1016/j.intimp.2018.04.016
Wojnicz D, Tichaczek-Goska D, Korzekwa K, Kicia M, Hendrich A (2017) Anti-enterococcal activities of pentacyclic triterpenes. Adv Clin Exp Med 26:483–490. https://doi.org/10.17219/acem/62245
Wu F, Bian D, Xia Y, Gong Z, Tan Q, Chen J, Dai Y (2012) Identification of major active ingredients responsible for burn wound healing of Centella asiatica herbs. Evid Based Complement Alternat Med 2012:848093. https://doi.org/10.1155/2012/848093
Wu S, Yang L, Sun W, Si L, Xiao S, Wang Q, Dechoux L, Thorimbert S, Sollogoub M, Zhou D et al (2017) Design, synthesis and biological evaluation of Gentiopicroside derivatives as potential antiviral inhibitors. Eur J Med Chem 130:308–319. https://doi.org/10.1016/j.ejmech.2017.02.028
Xiao W, Jiang W, Li K, Hu Y, Li S, Zhou L, Wan R (2017) Protective effect of Asiatic acid in an experimental cerulein-induced model of acute pancreatitis in mice. Am J Transl Res 9:1930–1934
Yamada H, Watanabe K, Saito T, Hayashi H, Niitani Y, Kikuchi T, Ito A, Fujikawa K, Lohmander LS (1999) Esculetin (dihydroxycoumarin) inhibits the production of matrix metalloproteinases in cartilage explants, and oral administration of its prodrug, CPA-926, suppresses cartilage destruction in rabbit experimental osteoarthritis. J Rheumatol 26:654–662
Yang L, Chen Q, Wang F, Zhang G (2011) Antiosteoporotic compounds from seeds of Cuscuta chinensis. J Ethnopharmacol 135:553–560. https://doi.org/10.1016/j.jep.2011.03.056
Yang SH, Liao CC, Chen Y, Syu JP, Jeng CJ, Wang SM (2012) Daidzein induces neuritogenesis in DRG neuronal cultures. J Biomed Sci 19:80. https://doi.org/10.1186/1423-0127-19-80
Yoo IH, Kim MJ, Kim J, Sung JJ, Park ST, Ahn SW (2019) The Anti-Inflammatory effect of Sulforaphane in mice with experimental autoimmune encephalomyelitis. J Korean Med Sci 34. https://doi.org/10.3346/jkms.2019.34.e197
Zeng S, Tai F, Zhai P, Yuan A, Jia R, Zhang X (2010) Effect of Daidzein on anxiety, social behavior and Spatial learning in male balb/cj mice. Pharmacol Biochem Behav 96:16–23. https://doi.org/10.1016/j.pbb.2010.03.015
Zhang Q, Zhang J, Xia P, Peng X, Li H, Jin H, Li Y, Yang J, Zhao L (2019) Anti-inflammatory activities of Gentiopicroside against iNOS and COX-2 targets. Chin Herb Med 11(1):108–112. https://doi.org/10.1016/j.chmed.2018.10.004
Zhang Y, Wu Q, Liu J, Zhang Z, Ma X, Zhang Y, Zhu J, Thring RW, Wu M, Gao Y, Tong H (2022) Sulforaphane alleviates high fat diet-induced insulin resistance via AMPK/Nrf2/GPx4 axis. Biomed Pharmacother 152:113273. https://doi.org/10.1016/j.biopha.2022.113273
Zhao CH, Xu J, Zhang YQ, Zhao LX, Feng B (2014) Inhibition of human enterovirus 71 replication by pentacyclic triterpenes and their novel synthetic derivatives. Chem Pharm Bull (Tokyo) 62:764–771. https://doi.org/10.1248/cpb.c14-00088
Zhao D, Shi Y, Dang Y, Zhai Y, Ye X (2015) Daidzein stimulates collagen synthesis by activating the TGF-β/smad signal pathway. Australas J Dermatol 56. https://doi.org/10.1111/ajd.12126
Zhou T, Zhu Y (2019) Cascade signals of Papaverine inhibiting LPS-Induced retinal microglial activation. J Mol Neurosci 68:111–119. https://doi.org/10.1007/s12031-019-01289-w
Author Information
Department of Zoology, Behali Degree College, Borgang, Biswanath, India