Allelopathic potential of Gloriosa superba L. rhizome leachate on Amaranthus viridis L., a weedy herb of India
*Article not assigned to an issue yet
Research Articles | Published: 03 September, 2025
First Page: 0
Last Page: 0
Views: 37
Keywords: Allelochemicals, Colchicine, Membrane permeability, Seed germination behavior, Seed priming, USL-01
Abstract
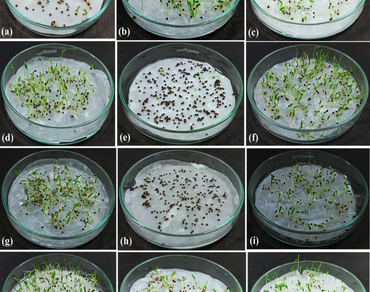
Allelopathy is an antagonistic activity exhibited by certain plants, enabling them to compete with neighboring species. The study aims to explore the allelopathic potential of the rhizome leachate (USL-01) of Gloriosa superba L. on the weedy species Amaranthus viridis L. by examining changes in seed germination behavior and biochemical parameters of A. viridis seedlings at different germination times. The effect of USL-01 (40 mg/ml, w/v) was compared with other chemicals like H2O2 (5%, v/v), kinetin (1 µg/ml, w/v), and colchicine (50.0 µg/ml, w/v), either alone or in combination. The seeds of A. viridis were primed with these chemicals for 6 h and 12 h, air-dried, and then used for germination assays. It was observed that the leachate USL-01, H2O2, and colchicine treatments caused significant impairment of seed germination, increased T50 values, and lowered the germination energy and seedling physio-biochemical parameters, including total carbohydrates, reduced sugars, amino acids, and total protein contents, in A. viridis seeds. It also increased the membrane permeability. At the same time, kinetin and its combination minimized the retardation factors of those treatments, equivalent to the untreated control. The study concludes that the USL-01 contains allelochemicals responsible for these growth effects, which interfere with the metabolism of sugars, amino acids, and proteins. Therefore, the compounds of USL-01, alone or in combination with H2O2 or colchicine, could be an excellent allelochemical agent in controlling weed species like A. viridis through 6-hrs and 12-hrs seed pretreatment.
References
Adhikary R, Bhattacharjee A, Mandal V (2018) Unplanned crop rotation and plantation affect seed health of crop plant: A case study. In: Defining modern biology: Plants and Microbes. Ed. Sarkar A, Mandal V, Sil SK, Majumder S, Barman C. Sujan Publication, India, pp 35–44. ISBN: 978-93-86564-02-3
Adhikary R, Mandal V (2019) Hydro-priming and hydration-dehydration treatment improve seed invigoration and biotic stress tolerance. Russ Agric Sci 45:35–42. https://doi.org/10.3103/S1068367419010129
Ain Q, Mushtaq W, Shadab M, Siddiqui MB (2023) Allelopathy: an alternative tool for sustainable agriculture. Physiol Mol Biol Plants 29(4):495–511. https://doi.org/10.1007/s12298-023-01305-9
Akl EM, El-Wakeel MA, Ahmed SA (2025) Potentiality of mustard seed extracts to reduce the biotic stress of weeds with enhancing the yield of wheat plants. Vegetos 26:1–2. https://doi.org/10.1007/s42535-025-01335-6
Al-Hawas GHS, Azooz MM (2018) Allelopathic potentials of Artemisia monosperma and Thymus vulgaris on growth and physio-biochemical characteristics of pea seedlings. Pak J Biol Sci 21(4):187–198
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studied. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Chauhan BS (2020) Grand challenges in weed management. Front Agron 1:3. https://doi.org/10.3389/fagro.2019.00003
Chauhan BS, Yadav A (2013) Weed management approaches for dry-seeded rice in india: a review. Ind J Weed Sci 45(1):1–6
Cheng F, Cheng Z (2015) Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front Plant Sci 6:1020. https://doi.org/10.3389/fpls.2015.01020
Chopra N, Tewari G, Tewari LM, Upreti B, Pandey N (2017) Allelopathic effect of Echinochloa colona L. and Cyperus Iria L. Weed extracts on the seed germination and seedling growth of rice and Soyabean. Adv Agri 2017:1–5. https://doi.org/10.1155/2017/5748524
Chou CH (1999) Methodologies for allelopathic research: from fields to laboratory. In: Macías FA, Galindo JCG, Molinillo JMG, Cutler HG (eds) Recent advances in allelopathy, vol. 1: A science for the future. International Allelopathy Society. Servicio De Publicaciones Universidad de Càdiz, Spain, pp 3–24
Chou CH (2010) Role of allelopathy in sustainable agriculture: use of allelochemicals as naturally occurring bio-agrochemicals. Allelopathy J 25:3–16
Choudhury PP, Singh R, Ghosh D, Sharma AR (2016) Herbicide use in Indian agriculture. ICAR - Directorate of Weed Research, Jabalpur, Madhya Pradesh, p 110
Cipollini K, Titus KYLE, Wagner C (2012) Allelopathic effects of invasive species (Alliaria petiolata, Lonicera maackii, Ranunculus ficaria) in the Midwestern united States. Allelopathy J 29(1):63–76
Darmanti S, Dewi K, Nugroho LH (2016) Antioxidative defenses of soybean [Glycine max (L.) Merr. cv. Grobogan] against purple nutsedge (Cyperus rotundus L.) interference during drought stress. JAPS: J Anim Plant Sci 26(1)
Dutra QP, Christ JA, Carrijo TT, de Assis Alves T, de Assis Alves T, Mendes LA, Praça-Fontes MM (2020) Phytocytotoxicity of volatile constituents of essential oils from Sparattanthelium mart. Species (Hernandiaceae). Sci Rep 10(1):12213. https://doi.org/10.1038/s41598-020-69205-6
Erb M, Kliebenstein DJ (2020) Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol 184(1):39–52. https://doi.org/10.1104/pp.20.00433
Fernandez C, Monnier Y, Santonja M, Gallet C, Weston LA, Prévosto B, Saunier A, Baldy V, Bousquet-Mélou A (2016) The impact of competition and allelopathy on the trade-off between plant defense and growth in two contrasting tree species. Front Plant Sci 7:594. https://doi.org/10.3389/fpls.2016.00594
Gharde Y, Singh PK, Dubey RP, Gupta PK (2018) Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot 107:12–18. https://doi.org/10.1016/j.cropro.2018.01.007
Gniazdowska A, Bogatek R (2005) Allelopathic interactions between plants. Multi-site action of allelochemicals. Acta Physiol Plant 27:395–407. https://doi.org/10.1007/s11738-005-0017-3
Grichi A, khammassi M, Khouja M, Amri I, Khouja ML (2025) Phytochemical screening and herbicidal properties of aqueous extract of Eucalyptus cinerea f.muell. Ex benth.: source of phenolic molecules with allelopathic potential. Vegetos 38:653–659. https://doi.org/10.1007/s42535-024-00815-5
Haig T (2008) Allelochemicals in plants. Allelopathy in sustainable agriculture and forestry. Springer, New York, NY, pp 63–104. https://doi.org/10.1007/978-0-387-77337-7_4
Hussain I, Singh NB, Singh A, Singh H (2017) Allelopathic potential of Sesame plant leachate against Cyperus rotundus L. Ann Agrarian Sci 15(1):141–147. https://doi.org/10.1016/j.aasci.2016.10.003
Hussain MI, El-Sheikh MA, Reigosa MJ (2020) Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 9(9):1228. https://doi.org/10.3390/plants9091228
Hussain MI, Reigosa MJ (2017) Evaluation of photosynthetic performance and carbon isotope discrimination in perennial ryegrass (Lolium Perenne L.) under allelochemical stress. Ecotoxicol (London England) 26(5):613–624. https://doi.org/10.1007/s10646-017-1794-3
Ismail BS, Tan PW, Chuah TS (2015) Assessment of the potential allelopathic effects of Pennisetum purpureum schumach. On the germination and growth of Eleusine indica (L.) Gaertn. Sains Malays 44(2):269–274
Janan AS, Eman RA, Fatin KI (2013) The effect of aqueous leaves extracts of Eucalyptus camaldulensis on germination and growth of three weed species. Rafidain J Sci 24(2):1–10
Jones DL, Owen AG, Farrar JF (2002) Simple method to enable the high-resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biol Biochem 34(12):1893–1902. https://doi.org/10.1016/S0038-0717(02)00203-1
Kantwa SR, Agrawal RK, Jha A, Pathan SH, Patil SD, Choudhary M, Roy AK (2019) Effect of different herbicides on weed control efficiency, fodder and seed yields of berseem (Trifolium Alexandrinum L.) in central India. Range Manage Agroforest 40(2):323–328
Kato-Noguchi H, Kurniadie D (2021) Allelopathy of Lantana Camara as an invasive plant. Plants (Basel Switzerland) 10(5):1028. https://doi.org/10.3390/plants10051028
Khatri K, Negi B, Bargali K, Bargali SS (2022) Spatial variation in allelopathic Inhibition by Ageratina adenophora on growth and yield of two traditional millet crops. Vegetos 35:663–673. https://doi.org/10.1007/s42535-022-00353-y
Kong CH, Xuan TD, Khanh TD, Tran HD, Trung NT (2019) Allelochemicals and signalling chemicals in plants. Molecules 24(15):2737. https://doi.org/10.3390/molecules24152737
Lebecque S, Crowet JM, Lins L, Delory BM, du Jardin P, Fauconnier ML, Deleu M (2018) Interaction between the barley allelochemical compounds Gramine and Hordenine and artificial lipid bilayers mimicking the plant plasma membrane. Sci Rep 8(1):9784. https://doi.org/10.1038/s41598-018-28040-6
Madhan Shankar R, Veeralakshmi S, Sirajunnisa AR, Rajendran R (2014) Effect of allelochemicals from leaf leachates of Gmelina Arborea on Inhibition of some essential seed germination enzymes in green gram, red gram, black gram, and Chickpea. Int Sch Res Notices 2014:1–7. https://doi.org/10.1155/2014/108682
Mangao AM, Arreola S, San Gabriel EV, Salamanez KC (2020) Aqueous extract from leaves of Ludwigia hyssopifolia (G. Don) Exell as a potential bioherbicide. J Sci Food Agric 100(3):1185–1194. https://doi.org/10.1002/jsfa.10128
Marinov-Serafimov P (2010) Determination of allelopathic effect of some invasive weed species on germination and initial development of grain legume crops. Pest Fitomed 25(3):251–259
Mekky MS, Hassanien AMA, Kamel EM, Ismail AEA (2019) Allelopathic effect of Ocimum Basilicum L. extracts on weeds and some crops and its possible use as new crude bio-herbicide. Ann Agric Sci 64(2):211–221. https://doi.org/10.1016/j.aoas.2019.12.005
Misra A, Srivastava S, Kumar S, Shukla PK, Kumar M, Agrawal PK, Barik SK (2020) Chemotaxonomic studies on natural population of Gloriosa Superba (L.) collected from gangetic plain (India) and their in vitro antigout activity for the identification of elite germplasm (s). J Ethnopharmacol 249:112387. https://doi.org/10.1016/j.jep.2019.112387
Mizutani J (1999) Selected allelochemicals. Crit Rev Plant Sci 18(5):653–671. https://doi.org/10.1080/07352689991309432
Moore S, Stein WH (1954) A modified ninhydrin reagent for photometric determination of amino acids and related compounds. J Biol Chem 211:907–913
Mubeen L, Nadeem MA, Tanveer A, Zahir ZA (2012) Allelopathic effects of sorghum and sunflower water extracts on germination and seedling growth of rice (Oryza sativa L.) and three weed species. J Anim Plant Sci 22(3):738–746
Muzell Trezzi M, Vidal RA, Balbinot Junior AA, von Hertwig Bittencourt H, da Silva Souza Filho AP (2016) Allelopathy: driving mechanisms governing its activity in agriculture. J Plant Interact 11(1):53–60. https://doi.org/10.1080/17429145.2016.1159342
Premaratna R, Weerasinghe MS, Premawardana NP, de Silva HJ (2015) Gloriosa Superba poisoning mimicking an acute infection- a case report. BMC Pharmacol Toxicol 16:27. https://doi.org/10.1186/s40360-015-0029-6
Puri S, Sidhu MC, Ahluwalia AS (2022) Establishing the dominating behavior of an aquatic plant ‘najas marina’ L. Vegetos 35:1069–1077. https://doi.org/10.1007/s42535-022-00383-6
Rao AN (2018) The historical and future perspective of Weed Science research in India. In: Fifty Years of Weed Research in India. Indian Society of Weed Science, pp. 1–23. ISBN 978-81-931978-7-5
Rao AN, Singh RG, Mahajan G, Wani SP (2020) Weed research issues, challenges, and opportunities in India. Crop Protect 134:104451. https://doi.org/10.1016/j.cropro.2018.02.003
Senthilkumar M (2013) Phytochemical screening of Gloriosa Superba L. - from different geographical positions. Int J Sci Res 3(1):1–5 ISSN 2250–3153
Sepat S, Thierfelder C, Sharma AR, Pavuluri K, Kumar D, Iquebal MA, Verma A (2017) Effects of weed control strategy on weed dynamics, soybean productivity, and profitability under conservation agriculture in India. Field Crops Res 210:61–70. https://doi.org/10.1016/j.fcr.2017.05.017
Singh AA, Rajeswari G, Nirmal LA, Jacob S (2021) Synthesis and extraction routes of allelochemicals from plants and microbes: A review. Rev Analyt Chem 40(1):293–311. https://doi.org/10.1515/revac-2021-0139
Singh V, Jat ML, Ganie ZA, Chauhan BS, Gupta RK (2016) Herbicide options for effective weed management in dry direct-seeded rice under scented rice-wheat rotation of Western Indo-Gangetic plains. Crop Protect 81:168–176. https://doi.org/10.1016/j.cropro.2015.12.021
Staszek P, Krasuska U, Ciacka K, Gniazdowska A (2021) ROS metabolism perturbation as an element of mode of action of allelochemicals. Antioxid (Basel Switzerland) 10(11):1648. https://doi.org/10.3390/antiox10111648
Tomar NS, Sharma M, Agarwal RM (2015) Phytochemical analysis of Jatropha Curcas L. during different seasons and developmental stages and seedling growth of wheat (Triticum aestivum L) as affected by extracts/leachates of Jatropha Curcas L. Physiol Molecul Biol Plants 21:83–92. https://doi.org/10.1007/s12298-014-0272-0
Umavathi S, Gopinath K, Manjula MS, Chinnasamy B, Ayyakannu A (2020) Gloriosa Superba L: a critical review of recent advances. Abasyn J Life Sci 3(2)
Weir TL, Park SW, Vivanco JM (2004) Biochemical and physiological mechanisms mediated by allelochemicals. Cur Opin Plant Biol 7(4):472–479. https://doi.org/10.1016/j.pbi.2004.05.007
Weston LA, Duke SO (2003) Weed and crop allelopathy. Crit Rev Plant Sci 22(3–4):367–389. https://doi.org/10.1080/713610861
Weston LA, Ryan PR, Watt M (2012) Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J Exp Bot 63(9):3445–3454. https://doi.org/10.1093/jxb/ers054
Whittaker RH, Feeny PP (1971) Allelochemics: chemical interactions between species: chemical agents are of major significance in the adaptation of species and organization of communities. Sci 171(3973):757–770. https://doi.org/10.1126/science.171.3973.757
Widiastuti H, Primaharinastiti P, Prihatiningtyas S (2014) Toxicity test from Gloriosa Superba L. leaves extract in rats (Rattus novegicus). Int J Pharm Pharm Sci 6(5):183–187
Yaduraju NT, Sharma AR, Rao AN (2015) Weeds in Indian agriculture: problems and prospects to become self-sufficient. Ind Farm 65(07):02–6
Yemm EW, Willis A (1954) The Estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508–514. https://doi.org/10.1042/bj0570508
Zhang X, Wang Z, Li H (2021) Allelopathic effects of Koelreuteria integrifoliola leaf aqueous extracts on lolium Perenne related to mesophyll ultrastructural alterations and endogenous hormone contents. Acta Physiol Plant 43:132. https://doi.org/10.1007/s11738-021-03303-4
Zhou Y, Yu J (2006) Allelochemicals and photosynthesis. In: Reigosa M, Pedrol N, González L (eds) Allelopathy. Springer, Dordrecht. https://doi.org/10.1007/1-4020-4280-9_6.
Zhu X, Yi Y, Huang L, Zhang C, Shao H (2021) Metabolomics reveals the allelopathic potential of the invasive plant Eupatorium adenophorum. Plants (Basel Switzerland) 10(7):1473. https://doi.org/10.3390/plants10071473
Author Information
Department of Botany, University of Gour Banga, Malda, India