Meta-topolin enhanced in vitro regeneration and genetic fidelity assessment of an endangered orchid, Pelatantheria insectifera using scot and ISSR
*Article not assigned to an issue yet
Research Articles | Published: 09 July, 2025
First Page: 0
Last Page: 0
Views: 155
Keywords: Orchidaceae, n Pelatantheria insectiferan , Protocorms, Regeneration, Micropropagation, Meta-topolin, Genetic stability
Abstract
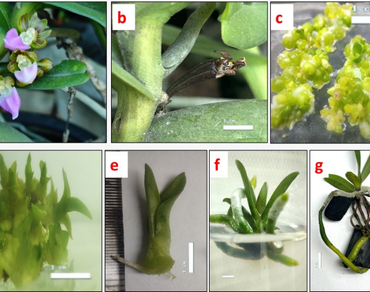
The present work focuses on the in vitro regeneration of Pelatantheria insectifera (Rchb.f.) Ridl, an endangered medicinal orchid, using meta-topolin (mT) and genetic fidelity to validate the multiplication strategy. This plant possesses a potential source of bioactive compounds that can be used for treating various illnesses associated with inflammatory conditions. Mass propagation of P. insectifera is important because of these properties, and it has also been listed as endangered under CITES due to habitat destruction and poor regeneration in the wild. The half-strength Murashige and Skoog’s (MS) medium supplemented with mT exhibited superior shoot growth compared to BAP. The optimal shoot proliferation (4.93 shoots per explant) occurred on the medium containing 1 mg/L mT, with the longest shoot (0.91 cm) found on the medium with 1.5 mg/L mT. The optimal number of leaves (3.30) was recorded on medium supplemented with 2 mg/L mT. The synergistic effect of mT or BAP combined with auxin (NAA) on shoot proliferation was assessed, with mT (1.5 mg/L) + NAA (0.5 mg/L) inducing maximum shoots per explant (17.66). Optimal in vitro rooting was achieved with 1.5 mg/L IBA, yielding the highest root count (6.10) and the greatest root length (7.02 cm). The clonal fidelity analysis with seven SCoT and five ISSR markers demonstrated 100% monomorphic banding patterns between the micropropagated plantlets and the mother plant. This simplified approach has the potential to propagate true-to-type planting material on a wide scale to reduce pressure on natural resources and restore natural populations of endangered commercially and medicinally valuable orchids.
References
Ahmad A, Anis M (2019) Meta-topolin improves in vitro morphogenesis, rhizogenesis and biochemical analysis in Pterocarpus marsupium roxb.: a potential drug-yielding tree. J Plant Growth Regul 38:1007–1016. https://doi.org/10.1007/s00344-018-09910-9
Ahmed MR, Anis M, Alatar AA, Faisal M (2017) In vitro clonal propagation and evaluation of genetic fidelity using RAPD and ISSR marker in micropropagated plants of Cassia alata L.: a potential medicinal plant. Agroforest Syst 91:637–647
Aremu AO, Bairu MW, Szüˇcová L, Doležal K, Finnie JF, van Staden J (2012) Assessment of the role of meta-topolins on in vitro produced phenolics and acclimatization competence of micropropagated Williams banana. Acta Physiol Plant 34:2265–2273. https://doi.org/10.1007/s11738-012-1027-6
Amom T, Tikendra L, Rahaman H, Potshangbam A, Nongdam P (2018) Evaluation of genetic relationship between 15 bamboo species of North-East India based on ISSR marker analysis. Mol Biol Res Commun 7:7–15. https://doi.org/10.22099/mbrc.2018.28378.1303
Bairu MW, Stirk WA, Doležal K, Van Staden J (2007) Optimizing the micropropagation protocol for the endangered Aloe polyphylla: can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tissue Organ Cult 90:15–23
Bhattacharyya P, Kumar V, Van Staden J (2017) Assessment of genetic stability amongst micropropagated Ansellia africana, a vulnerable medicinal Orchid species of Africa using scot markers. South Afr J Bot 108:294–302. https://doi.org/10.1016/j.sajb.2016.11.007
Bhattacharyya P, Kumaria S, Diengdoh R, Tandon P (2014) Genetic stability and phytochemical analysis of the in vitro regenerated plants of Dendrobium nobile lindl., an endangered medicinal Orchid. Meta Gene 2:489–504
Bose B, Kumaria S, Choudhury H, Tandon P (2017) Insights into nuclear DNA content, hydrogen peroxide and antioxidative enzyme activities during transverse thin cell layer organogenesis and ex vitro acclimatization of Malaxis wallichii, a threatened medicinal Orchid. Physiol Mol Biology Plant 23:955–968
Chahal S, Kaur H, Lekhak MM, Shekhawat MS, Goutam U, Singh SK, Ochatt SJ, Kumar V (2022) Meta-topolin-mediated regeneration and accumulation of phenolic acids in the critically endangered medicinal plant Crinum Malabaricum (Amaryllidaceae): A potent source of Galanthamine. South Afr J Bot 149:853–859. https://doi.org/10.1016/j.sajb.2022.01.016
Chauhan RD, Taylor NJ (2018) Meta-topolin stimulates de Novo shoot organogenesis and plant regeneration in cassava. Plant Cell Tissue Organ Cult 132:219–224
Chatterjee G, Prakash J (1996) In: Prakash J, Pierik RLM (eds) Genetic stability in commercial tissue culture. Oxford IBH Publishing Co, New Delhi
Chowlu K, Angela N, Rao AN, Vij SP (2013) Coelogyne fuscescens var. Brunnea (Lindley) Lindley [Orchidaceae] – a new record to manipur, India. Pleione 7(1):275–278
Collard BCY, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biology Report 27:86–93
Elayaraja D, Subramanyam K, Vasudevan V, Sathish S, Kasthurirengan S, Ganapathi A, Manickavasagam M (2019) Meta-Topolin (mT) enhances the in vitro regeneration frequency of Sesamum indicum (L). Biocatal Agric Biotechnol 21:101320. https://doi.org/10.1016/j.bcab.2019.101320
Fatahi M, Vafaee Y, Nazari F, Tahir NAR (2022) In vitro asymbiotic seed germination, protocorm formation, and plantlet development of Orchis Simia lam.: A threatened terrestrial Orchid species. S Afr J Bot 151:156–165. https://doi.org/10.1016/j.sajb.2022.09.03
Gholami S, Vafaee Y, Nazari F et al (2021) Exploring genetic variations in threatened medicinal orchids using start codon targeted (SCoT) polymorphism and marker-association with seed morphometric traits. Physiol Mol Biol Plants 27:769–785. https://doi.org/10.1007/s12298-021-00978-4
Goto S, Thakur RC, Ishii K (1998) Determination of genetic stability in long-term micropropagated shoots of Pinus thunbergii Parl using RADP markers. Plant Cell Rep 18:193–197. https://doi.org/10.1007/s002990050555
Guo B, Chen H, Yin Y, Wang W, Zeng S (2024a) Tissue culture via protocorm-like bodies in an orchids hybrids Paphiopedilum SCBG Huihuang90. Plants 13(197). https://doi.org/10.3390/plants13020197
Guo B, Chen H, Yin Y, Wang W, Zeng S (2024b) Tissue culture via protocorm-like bodies in an orchids hybrids Paphiopedilum SCBG Huihuang90. Plants 13(2):197. https://doi.org/10.3390/plants13020197
Guo B, Zeng S, Yin Y et al (2021) Characterization of phytohormone and transcriptome profiles during protocorm-like bodies development of Paphiopedilum. BMC Genomics 22:806. https://doi.org/10.1186/s12864-021-08087-y
Haisel D, Hofman P, Vágner M, Lipavská H, Tichá I, Schäfer C, Čapková V (2001) Ex vitro phenotype stability is affected by. Vitro Cultivation Biol Plant 44:321–324. https://doi.org/10.1023/A:1012415004676
Hoque MM, Huda MK, Eva TA (2021) Pharmacological and phytochemical profile of an endangered epiphytic orchid, Pelatantheria insectifera (Rchb.f.) ridl. J Orchid Soc India 35:1–7
Kaladharan S, Rengasamy A, Chinnaiyan R, Mariappan M, Thiruppathi SK (2024) In vitro asymbiotic seed germination and micropropagation of Dendrobium heyneanum Lindl.–an endemic Orchid of Western ghats, India. Plant Cell Tissue Organ Cult (PCTOC) 157(2):1–2
Kanlayavattanakul M, Lourith N (2022) Orchid extracts and cosmetic benefits. Orchids phytochemistry, biology and horticulture: fundamentals and applications. Springer International Publishing, Cham, pp 609–626. https://doi.org/10.1007/978-3-030-38392-3_22
Khanam MN, Javed SB, Anis M, Alatar AA (2019) meta-Topolin induced in vitro regeneration and metabolic profling in Allamanda cathartica L. Ind Crops Prod 145. https://doi.org/10.1016/j.indcrop.2019.111944
Kishor R, Chowlu K, Vij SP (2012) Ione kipgenii (Orchidaceae), a new species from manipur, India. Kew Bull 67:517–519
Koç E (2021) Effects of Meta-topolin on the growth, physiological and biochemical parameters in plant tissue culture. In: Ahmad N, Strnad M (eds) Meta-topolin: A growth regulator for plant biotechnology and agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-15-9046-7_19
Li C, Dong N, Zhao Y, Wu S, Liu Z, Zhai J (2021) A review for the breeding of orchids: current achievements and prospects. Hortic Plant J 7(5):380–392. https://doi.org/10.1016/j.hpj.2021.02.006
Magyar-Tábori K, Dobránszki J, Jámbor-Benczúr E, Bubán T, Lazányi J, Szalai J, Ferenczy A (2001) Post- effects of cytokinins and auxin levels of proliferation media on rooting ability of in vitro Apple shoots (Malus domestica Borkh.) ‘red fuji’. Int J Hort Sci 7:26–29
Manokari M, Latha R, Priyadharshini S, Jogam P, Shekhawat MS (2020) Short-term cold storage of encapsulated somatic embryos and retrieval of plantlets in grey orchid (Vanda tesellata (Roxb.) Hook. Ex G. Don). Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-020-01899-y
Manokari M, Priyadharshini S, Jogam P, Dey A, Shekhawat MS (2021) Meta-topolin and liquid medium mediated enhanced micropropagation via Ex vitro rooting in Vanilla planifolia jacks. Ex Andrews. Plant Cell Tiss Organ Cult 146:69–82
Naidoo D, Aremu AO, Van Staden J, Finnie JF (2017) In vitro plant regeneration and alleviation of physiological disorders in Scadoxus puniceus. S Afr J Bot 109:316–322. https://doi.org/10.1016/j.sajb.2017.01.010
Nongdam P, Beleski DG, Tikendra L, Dey A, Varte V, Merzougui EL, Pereira S, Barros VM, Vendrame PR WA (2023) Orchid micropropagation using conventional Semi-Solid and temporary immersion systems: A review. Plants 12:1136. https://doi.org/10.3390/plants12051136
Nowakowska K, Pacholczak A (2020) Comparison of the effect of Meta-Topolin and benzyladenine during Daphne mezereum L. Micropropagation. Agronomy 10(1994). https://doi.org/10.3390/agronomy10121994
Palombi M, Damiano C (2002) Comparison between RAPD and SSR molecular markers in detecting genetic variation in Kiwifruit (Actinidia deliciosa A. Chev). Plant Cell Rep 20:1061–1066
Parthibhan S, Rao MV, Kumar TS (2015) In vitro regeneration from protocorms in Dendrobium aqueum Lindley-an imperilled Orchid. J Genet Engg Biotechnol 13:227–233. https://doi.org/10.1016/j.jgeb.2015.07.001
Pathak P, Kumari A, Chandler BD, Zettler LW (2023) In vitro propagation and phytochemical analysis of Vanda Cristata wall. Ex lindl: an endangered medicinal Orchid of biopharmaceutical importance. S Afr J Bot 153:109–123. https://doi.org/10.1016/j.sajb.2022.11.023
Poeaim A, Kunwanlop W, Boonmee W, Laipasu P (2022) Effects of 6- benzylaminopurine and meta-topoline on micropropagation of Dendrobium Chrysanthum Lindl. Int J Agricultural Technol 18(5):2185–2192
Pradhan S, Tiruwa BL, Subedee BR, Pant B (2016) Efficient plant regeneration of Cymbidium aloifolium (L.) sw., a threatened Orchid of Nepal through artificial seed technology. Am J Plant Sci 7:1964–1974. https://doi.org/10.4236/ajps.2016.714179
Pramanik D, Yufdy MP, Shintiavira H, Winarto B (2016) In vitro secondary embryogenesis derived from meta-topoline treatment on mass propagation of Phalaenopsis ‘AMP 17’. Notulae Scientia Biologicae 8(1):62–68
Priyadharshini S, Kannan N, Manokari M, Shekhawat MS (2021) In vitro regeneration using twin scales for restoration of critically endangered aquatic plant Crinum Malabaricum Lekhak & yadav: a promising source of Galanthamine. Plant Cell Tis Sue Organ Cult 141:593–604
Rao AN (2007) Orchid flora of North East India – An upto date analysis. Bull Arunachal Res 23(1):6–38
Sharma A, Pathak P (2020) The budding potential of orchids in the cosmeceutical sector: role of orchids in skincare and health. J Orchid Soc 34:79–85
Teklehaymanot T, Wannakrairoj S, Pipattanawong N (2010) Meta-topolin for pineapple shoot multiplication under three in vitro systems. Am Euras J Agr Environ Sci 7:157–162
Teoh ES (2021) Pelatantheria ridl. Orchid species from himalaya and Southeast Asia. G - P), vol 2. Springer, Cham, pp 235–237. https://doi.org/10.1007/978-3-030-80428-2_31
Thakur M, Sharma V, Chauhan A (2021) Genetic fidelity assessment of long term in vitro shoot cultures and regenerated plants in Japanese Plum Cvs Santa Rosa and frontier through RAPD, ISSR and scot markers. South Afr J Bot 140:428–433
Tikendra L, Koijam AS, Nongdam P (2019) Molecular markers based genetic fidelity assessment of micropropagated Dendrobium chrysotoxum Lindl. Meta Gene 20:100562. https://doi.org/10.1016/j.mgene.2019.100562
Tikendra L, Potshangbam AM, Dey A et al (2021) RAPD, ISSR, and scot markers based genetic stability assessment of micropropagated Dendrobium fimbriatum lindl. Var. Oculatum hk. f.- an important endangered Orchid. Physiol Mol Biol Plants 27:341–357. https://doi.org/10.1007/s12298-021-00939-x
Tinoammini N, Aazhivaendhan G, Kumar TS (2024) In vitro germination and optimization of basal media for protocorm-like bodies proliferation in Dienia ophrydis (J. Koenig) Seidenf. Rhizosphere 29:100854. https://doi.org/10.1016/j.rhisph.2024.100854
Tiwari P, Sharma A, Bose SK, Park KI (2024) Advances in Orchid biology: biotechnological achievements, translational success, and commercial outcomes. Hortic 10(2):152. https://doi.org/10.3390/horticulturae10020152
Valero-Aracama C, Kane M, Wilson S, Philman N (2010) Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult and easy to acclimatize sea Oats (Uniola paniculata L.) genotypes. Plant Growth Regul 60:43–49
van der Walt K, Lehnebach CA, Alderton-Moss J (2024) Asymbiotic germination, seedling establishment and fungal uptake of Pterostylis Montana and P. paludosa, two Orchid species endemic to new Zealand. N Z J Bot 31:1–8
Author Information
Institute of Bioresources and Sustainable Development (IBSD), Takyelpat, India