Two amino-acid polymorphisms in PBP4 generate penicillin tolerance in group B streptococcus
Kim H., Fittipaldi B., Hoque F., Wang C., Zefi O., Li W., Goldman Z., Peter Y., Basu P., Fittipaldi B.
Research Articles | Published: 30 September, 2022
First Page: 106
Last Page: 118
Views: 3026
Keywords: Antibiotic tolerance, d-Alanyl-d-alanine carboxypeptidases Pbp4, Group B streptococcus, Mechanisms of tolerance
Abstract
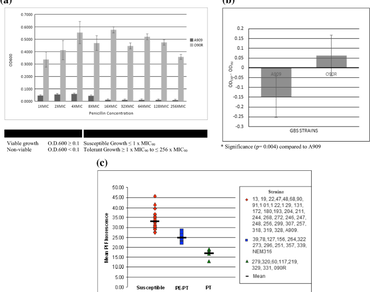
Group B streptococci (GBS) bacteria are the leading cause of neonatal infections in the US. GBS strains may exhibit prolonged resilience to clinical treatments, becoming antibiotic tolerant. Penicillin tolerance (PT) in many Gram-positive bacteria is associated with the highly conserved penicillin-binding protein 4 (pbp4). To determine potential amino acid polymorphisms (AAP) associated with PT in GBS, we characterized the pbp4 gene of 48 clinical isolates. Defining PT as a detectable growth of ≥ 1× MIC90 in the penicillin killing and penicillinase regrowth assay, 7/48 (14.6%) of the strains were PT, and 11/48 (22.9%) were characterized as paradoxical-PT (PE–PT). Sequence alignment of pbp4, identified two AAPs, previously found to be in linkage disequilibrium, G168D, and V289I, that were associated with the PT strains. Within the isolates, the mutant pbp4 allele (pbp4mu) was found in 0/30 (0%) of susceptible, as opposed to 5/7 (71.4%) of PT and 7/11 (63.7%) of PE–PT samples (χ2 = 40.1; df = 2; P < 0.001). To confirm the effect of the G168D and V289I substitutions in GBS, we replaced the wild-type pbp4 (pbp4wt) in a susceptible strain (A909) with pbp4mu and substituted the pbp4mu with pbp4wt in a PT strain (090R). Prevalence of pbp4mu in A909 stabilized cell wall peptidoglycan cross-linkage, decreased membrane damage, and penicillin-mediated killing increasing regrowth tolerance. The reverse was seen upon expression of pbp4wt in 090R. Our findings indicate that AAPs in the penicillin-binding protein 4 of GBS generated by two novel single nucleotide polymorphisms (SNPs) in the pbp4 gene are associated with PT and that the occurrence of one or both mutations can serve as biomarkers used in real-time PCR-based screening assays for detection of PT in GBS and consequently improve treatment guidelines.
References
Betriu C et al (1989) Penicillin tolerance of group A streptococci. Eur J Clin Microbiol Infect Dis 8:799–800
Betriu C et al (1994) Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother 38:2183–2186
Bradely JJ, Mayhall CG, Dalton HP (1978) Incidence and characteristics of antibiotic-tolerant strains of Staphylococcus aureus. Antimicrob Agents Chemother 13:1052–1057
Brown WJ (1988) National Committee for Clinical Laboratory Standards agar dilution susceptibility testing of anaerobic gram-negative bacteria. Antimicrob Agents Chemother 32(3):385–390
Chaussee MA, McDowell EJ, Rieck LD, Callegari EA, Chaussee MS (2006) Proteomic analysis of a penicillin-tolerant rgg mutant strain of Streptococcus pyogenes. J Antimicrob Chemother 58:752–759
Dahesh S et al (2008) Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob Agents Chemother 52:2915–2918
de Jonge BL, Chang YS, Gage D, Tomasz A (1992) Peptidoglycan composition in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Biol Chem 267:11255–11259
Dörr T (2021) Understanding tolerance to cell wall-active antibiotics. Ann NY Acad Sci 1496(1):35–58. https://doi.org/10.1111/nyas.14541
Eagle H, Musselman AD (1948) The rate of bactericidal action of penicillin in vitro as a function of its concentration, and its paradoxically reduced activity at high concentrations against certain organisms. J Exp Med 88:99–131
Framson PE, Nittayajarn A, Merry J, Youngman P, Rubens CE (1997) New genetic techniques for group B streptococci: high-efficiency transformation, maintenance of temperature-sensitive pWV01 plasmids, and mutagenesis with Tn917. Appl Environ Microbiol 63:3539–3547
Goffin C, Ghuysen JM (2002) Biochemistry and comparative genomics of SxxK superfamily acyltransferases offer a clue to the mycobacterial paradox: presence of penicillin-susceptible target proteins versus lack of efficiency of penicillin as therapeutic agent. Microbiol Mol Biol Rev 66:702–738 table of contents
Haas W, Sublett J, Kaushal D, Tuomanen EI (2004) Revising the role of the pneumococcal vex-vncRS locus in vancomycin tolerance. J Bacteriol 186:8463–8471
Haas W, Kaushal D, Sublett J, Obert C, Tuomanen EI (2005) Vancomycin stress response in a sensitive and a tolerant strain of Streptococcus pneumoniae. J Bacteriol 187:8205–8210
Haenni M, Majcherczyk PA, Barblan JL, Moreillon P (2006) Mutational analysis of class A and class B penicillin-binding proteins in Streptococcus gordonii. Antimicrob Agents Chemother 50(12):4062–4069. https://doi.org/10.1128/AAC.00677-06
Handwerger S, Tomasz A (1985) Antibiotic tolerance among clinical isolates of bacteria. Rev Infect Dis 7:368–386
Hanslik T, Hartig C, Jurand C, Armand-Lefevre L, Jubault V, Rouveix E, Dubourg O, Prinseau J, Baglin A, Nicolas-Chanoine MH (2003) Clinical significance of tolerant strains of streptococci in adults with infective endocarditis. Clin Microbiol Infect 9(8):852–857
Hayes K, O’Halloran F, Cotter L (2020) A review of antibiotic resistance in group B streptococcus: the story so far. Crit Rev Microbiol 46(3):253–269. https://doi.org/10.1080/1040841X.2020.1758626
Ishida K, Guze PA, Kalmanson GM, Albrandt K, Guze LB (1982) Variables in demonstrating methicillin tolerance in Staphylococcus aureus strains. Antimicrob Agents Chemother 21:688–690
James PA (1990) Comparison of four methods for the determination of MIC and MBC of penicillin for viridans streptococci and the implications for penicillin tolerance. J Antimicrob Chemother 25:209–216
Kern J, Ryan C, Faull K, Schneewind O (2010) Bacillus anthracis surface-layer proteins assemble by binding to the secondary cell wall polysaccharide in a manner that requires csaB and tagO. J Mol Biol 401(5):757–775
Koch AL (2001) Autolysis control hypotheses for tolerance to wall antibiotics. Antimicrob Agents Chemother 45:2671–2675
Mamou G, Corona F, Cohen-Khait R, Housden NG, Yeung V, Sun D, Sridhar P, Pazos M, Knowles TJ, Kleanthous C, Vollmer W (2022) Peptidoglycan maturation controls outer membrane protein assembly. Nature 606(7916):953–959
Murdoch DR, Reller LB (2001) Antimicrobial susceptibilities of group B streptococci isolated from patients with invasive disease: 10-year perspective. Antimicrob Agents Chemother 45(12):3623–3624
Pazos M, Vollmer W (2021) Regulation and function of class A penicillin-binding proteins. Curr Opin Microbiol 60:80–87. https://doi.org/10.1016/j.mib.2021.01.008
Phares CR et al (2008) Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065
Popham DL, Young KD (2003) Role of penicillin-binding proteins in bacterial cell morphogenesis. Curr Opin Microbiol 6(6):594–599. https://doi.org/10.1016/j.mib.2003.10.002
Raabe VN, Shane AL (2019) Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr 7(2). 10.1128. /microbiolspec.GPP3-0007-2018
Regan JA, Klebanoff MA, Nugent RP (1991) The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet Gynecol 77:604–610
Robertson GT et al (2002) Vancomycin tolerance induced by erythromycin but not by loss of vncRS, vex3, or pep27 function in Streptococcus pneumoniae. J Bacteriol 184:6987–7000
Sader HS, Fritsche TR, Jones RN (2006) Daptomycin bactericidal activity and correlation between disk and broth microdilution method results in testing of Staphylococcus aureus strains with decreased susceptibility to vancomycin. Antimicrob Agents Chemother 50:2330–2336
Saribas S, Bagdatli Y (2004) Vancomycin tolerance in enterococci. Chemotherapy 50:250–254
Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P (2008) The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev 32(2):234–258. https://doi.org/10.1111/j.1574-6976.2008.00105.x
Scheffers DJ, Pinho MG (2005) Bacterial cell wall synthesis: new insights from localization studies. Microbiol Mol Biol Rev 69:585–607
Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A (2002) Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 51:1–22
Sherris JC (1986) Problems in in vitro determination of antibiotic tolerance in clinical isolates. Antimicrob Agents Chemother 30:633–637
Steininger C, Allerberger F, Gnaiger E (2002) Clinical significance of inhibition kinetics for Streptococcus pyogenes in response to penicillin. J Antimicrob Chemother 50:517–523
van Asselt GJ, de Kort G, van de Klundert JA (1995) Differences in penicillin- binding protein patterns of penicillin tolerant and non-tolerant group A streptococci. J Antimicrob Chemother 35:67–74
van der Meer JT, van Vianen W, Hu E, van Leeuwen WB, Valkenburg HA, Thompson J, Michel MF (1991) Distribution, antibiotic susceptibility and tolerance of bacterial isolates in culture-positive cases of endocarditis in The Netherlands. Eur J Clin Microbiol Infect Dis 10(9):728–734
Wallis RS et al (1999) Drug tolerance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 43:2600–2606
Westblade LF, Errington J, Dörr T (2020) Antibiotic tolerance. PLoS Pathog 16(10):e1008892. https://doi.org/10.1371/journal.ppat.1008892
Yocum RR, Rasmussen JR, Strominger JL (1980) The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. J Biol Chem 255(9):3977–3986
Zhou Y, Antignac A, Wu SW, Tomasz A (2008) Penicillin-binding proteins and cell wall composition in beta-lactam-sensitive and -resistant strains of Staphylococcus sciuri. J Bacteriol 190(2):508–514
Rice KC, Bayles KW (2008) Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev 72(1):85–109. https://doi.org/10.1128/MMBR.00030-07
Author Information
Touro College of Pharmacy, New York, USA