Survey and characterization of cabbage head rot incited by Sclerotinia sclerotiorum from major crucifers growing areas of Tamil Nadu
*Article not assigned to an issue yet
Research Articles | Published: 26 March, 2025
First Page: 0
Last Page: 0
Views: 834
Keywords: Cabbage, Head rot, n Sclerotinia sclerotiorumn , Survey, Molecular characterization
Abstract
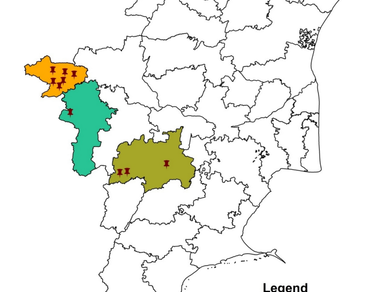
Cabbage (Brassica oleracea var. capitata) is one of the most popular winter vegetable crops across the world. The cultivation of cabbage is severely hindered by various diseases. Among them, “head rot or white mold” is a potential threat to cabbage cultivation and production. Head rot is the only disease that occurs both in the field as well as in storage conditions. A survey was conducted during 2019–2020 in major crucifers growing areas of Tamil Nadu. The disease incidence was recorded in all the major districts such as Narasipuram village of Coimbatore district, Ketti, Muthorai, Emerald, Palada, Kappathorai and Nanjanadu village of The Nilgiri district, Poombarai, Vilpatti and Thadiyankudisai area of Dindigul district surveyed. The highest incidence (72.1%) was recorded in the Nanjanadu area of the Nilgiris district. The lowest incidence (10.1%) was recorded in Narasipuram village of Coimbatore district. The pathogen was identified as Sclerotinia sclerotiorum morphologically as well as molecularly using specific (SSFWD and SSREV) universal primers (ITS 1 and 4) with an amplicon size of approximately 280 bp and 580 bp respectively. All the isolates were sequenced and using accession numbers the phylogenetic tree was constructed to understand the genetic relationships among the isolates.
References
Alagianagalingam MN, Mohan R, Subramanian CL, Sampanthamoorthy S (1978) A new record of sclerotinia rot of cabbage in India. Current Science.
Anonymous. 2018. National Horticulture Board, India.
Anonymous. 2019. Horticultural Statistics Division, DAC&FW.
Bolton MD, Thomma BPHJ, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol. 7(1):1–16. https://doi.org/10.1111/j.1364-3703.2005.00316.x
Borah TR, Dutta S, Barman AR (2018) First report of Sclerotinia sclerotiorum on Mimosa pudica in India. New Dis Rep 38(14):2044–588. https://doi.org/10.5197/j.2044-0588.2018.038.014
Chakraborty BN, Chakraborty U, Saha A, Dey PL, Sunar K (2010) Molecular characterization of Trichoderma viride and Trichoderma harzianum isolated from soils of North Bengal based on rDNA markers and analysis of their PCR-RAPD profiles. Global J Biotechnol Biochem 5(1):55–61
Danielson GA, Nelson BD, Helms TC (2004) Effect of Sclerotinia stem rot on yield of soybean inoculated at different growth stages. Plant Dis 88(3):297–300. https://doi.org/10.1094/PDIS.2004.88.3.297
Duttay S (2009) First report of Sclerotinia stem rot of Indian sweet basil (Ocimum basilicum). https://doi.org/10.1007/s13314-024-00540-7
Freeman J, Ward E, Calderon C, McCartney A (2002) A polymerase chain reaction (PCR) assay for the detection of inoculum of Sclerotinia sclerotiorum. Eur J Plant Pathol 108(9):877–886. https://doi.org/10.1023/A:1021216720024
Gupta AK, Bashyal BM, Choudhary R, Kumar M, Solanki IS (2015) First report of Sclerotinia rot of pigeonpea caused by Sclerotinia sclerotiorum (Lib.) de Bary in India. Can J Plant Pathol 37(4):514–518. https://doi.org/10.1080/07060661.2015.1115782
Hossain MM, Sultana F, Rubayet MT, Khan S, Mostafa M, Mishu NJ, Sabbir MAA, Akter N, Kabir A, Mostofa MG (2024) White mold: a global threat to crops and key strategies for its sustainable management. Microorganisms 13(1):4. https://doi.org/10.3390/microorganisms13010004
Krishnamoorthy KK, Sankaralingam A, Nakkeeran S (2017) Management of head rot of cabbage caused by Sclerotinia sclerotiorum through combined application of fungicides and biocontrol Bacillus amyloliquefaciens. IJCS 5(2):401–404
Kumar SV, Rajeshkumar P, Senthilraja C, Nakkeran S (2015) First report of Sclerotinia sclerotiorum causing stem rot of carnation (Dianthus caryophyllus) in India. Plant Dis 99:1280. https://doi.org/10.1094/PDIS-02-15-0240-PDN
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Loganathan M, Sible GV, Maruthasalam S, Saravanakumar D, Raguchander T, Sivakumar M, Samiyappan R (2010) Trichoderma and chitin mixture-based bioformulation for the management of head rot (Sclerotinia sclerotiorum (Lib.) de Bary)–root-knot (Meloidogyne incognita Kofoid and White; Chitwood) complex diseases of cabbage. Arch Phytopathol Plant Prot 43(10):1011–1024. https://doi.org/10.1080/03235400802214885
Prasad L, Kamil D, Solanki RK, Singh B (2017) First report of Sclerotinia sclerotiorum infecting cumin (Cuminum cyminum) in India. Plant Dis 101(5):833–833. https://doi.org/10.1094/PDIS-08-16-1089-PDN
Rai D, Ranjan RK, Dwivedi M (2024) Detection of Sclerotinia rot of basil, caused by Sclerotinia sclerotiorum for the first time in Bihar. India Aust Plant Dis Notes 19(1):1–3. https://doi.org/10.1007/s13314-024-00540-7
Ramsey GB, Wiant J, Link GKK (1938) Market diseases of fruits and vegetables: Crucifers and cucurbits. U.S. Department of Agriculture.
Rathi AS, Jattan M, Punia R, Singh S, Kumar P, Avtar R (2018) Morphological and molecular diversity of Sclerotinia sclerotiorum infecting Indian mustard. Indian Phytopathol 71(3):407–413. https://doi.org/10.1007/s42360-018-0054-7
Saharan GS, Mehta N (2008) Sclerotinia diseases of crop plants: Biology, ecology and disease management. Springer Sci Bus Med. https://doi.org/10.1007/978-1-4020-8408-9
Sambrook HC (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor.
Singh H, Kalda TS (1995) Screening of cauliflower germplasm against Sclerotinia rot. Indian J Genet Plant Breed 55(1):98–102
Sinha AK, Sahu S, Dwivedi H, Bandamaravuri KB (2024) Stem rot disease of Withania somnifera caused by Sclerotinia sclerotiorum in India. Aust Plant Dis Notes 19(1):22. https://doi.org/10.1007/s13314-024-00545-2
Tripathi AN, De RK, Sharma HK, Karmakar PG (2015) Emerging threat of Sclerotinia sclerotiorum causing white/cottony stem rot of mesta in India. New Dis Rep 32(19):2044–588. https://doi.org/10.5197/j.2044-0588.2015.032.019
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols Guide Methods Appl 18(1):315–322
Willetts HJ, Wong JAL (1980) The biology of Sclerotinia sclerotiorum, S. trifoliorum, and S. minor with emphasis on specific nomenclature. Bot Rev 46(2):101–165. https://doi.org/10.1007/BF02860868
Yadav S, Srivastava AK, Singh DP, Arora DK (2012) Isolation of oxalic acid-tolerating fungi and decipherization of its potential to control Sclerotinia sclerotiorum through oxalate oxidase-like protein. World J Microbiol Biotechnol 28(11):3197–3206. https://doi.org/10.1007/s11274-012-1130-2
Author Information
Department of Plant Pathology, Centre for Plant Protection Studies, Tamil Nadu Agricultural University, Coimbatore, India