Stenotrophomonas: a versatile diazotrophic bacteria from the rhizospheric soils of Western Himalayas and development of its liquid biofertilizer formulation
Research Articles | Published: 06 March, 2019
First Page: 103
Last Page: 109
Views: 4170
Keywords: Liquid carriers, Nitrogenase, PGPR, Rhizosphere, 16S rRNA gene, Stenotrophomonas rhizophila
Abstract
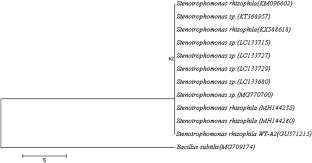
Rhizosphere is a rich repository of plant growth promoting rhizobacteria (PGPR) which is a sustainable tool to increase crop productivity and maintain soil health. In this context, 43 isolates were obtained on Jensen’s medium from the rhizosphere of Triticum aestivum, Zea mays, Solanum tuberosum, Aloe barbadensis and Bacopa monnieri grown in Palampur, (Himachal Pradesh) India. Out of these isolates, only six isolates (WT-A2, WT-A1, MZ-A2, PT-A1, PT-A3 and BM-A3) exhibited significantly higher nitrogenase activity (451.45, 441.58, 440.91, 444.02, 383.64 and 374.44 nmole C2H4 h−1 mg−1 protein) as compared to the reference strain of Azotobacter chroococum MTCC 446 (372.85 nmole C2H4 h−1 mg−1 protein). The isolate WT-A2 was the most efficient with respect to nitrogenase activity (451.45 nmole C2H4 h−1 mg−1 protein), indole acetic acid production (17.45 μg ml−1), ammonia production and siderophore production. Isolate WT-A2 was identified as Stenotrophomonas rhizophila on the basis of morphological, biochemical and 16S rRNA sequence analysis. In order to prepare liquid bioinoculant formulation, survivability studies on S. rhizophila was carried out in four different liquid carriers (Compost Tea, Biogas slurry, Vermiwash and Minimal Growth Medium) at room temperature (average maximum temp. was 23.83 °C and average minimum temp. was 11.91 °C). The results showed that S. rhizophila survived better in different liquid carriers (9.873 log cfu ml−1 in biogas slurry; 9.843 log cfu ml−1 in vermiwash; 9.163 log cfu ml−1 in minimal growth medium), and Compost Tea was the best carrier to support higher bacterial load (9.907 log cfu ml−1) on 180th day of storage. The results are of practical importance as this (compost tea) liquid carrier could be used to produce liquid biofertilizer formulation. Also, S. rhizophila could be a potential biofertilizer candidate as it posses multifarious plant growth promoting traits.
References
- Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Uni-Sci 26:1–20. https://doi.org/10.1016/j.jksus.2013.05.001
- Andrade G, Esteban E, Velascol L, Maria JL, Bedmar EJ (1997) Isolation and identification of N2-fixing microorganisms from the rhizosphere of Capparis spinosa (L.). Plant Soil 197:19–23. https://doi.org/10.1023/A:1004211909641
- Asano Y, Lubbehusen TL (2000) Enzymes acting on peptides containing d-amino acid. J Biosci Bioeng 89:295–306. https://doi.org/10.1016/S1389-1723(00)88949-5
- Bakker AW, Schippers P (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp.-mediated plant growth-stimulation. Soil Biol Biochem 19:451–457. https://doi.org/10.1016/0038-0717(87)90037-X
- Banerjee M, Yesmin L (2002) Sulfur-oxidizing plant growth promoting rhizobacteria for enhanced canola performance. US Patent 07491535
- Bashan Y (1998) Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv 16:729–770. https://doi.org/10.1016/S0734-9750(98)00003-2
- Basu S, Rabara RC, Negi S, Shukla P (2018) Engineering PGPMOs through gene editing and systems biology: a solution for phytoremediation? Trends Biotechnol 36:499–510. https://doi.org/10.1016/j.tibtech.2018.01.011
- Benizri E, Courtade A, Picard C, Guckert A (1998) Role of maize root exudates in the production of auxins by Pseudomonas fluorescens M.3.1. Soil Biol Biochem 30:1481–1484. https://doi.org/10.1016/S0038-0717(98)00006-6
- Blomberg A, Adler L (1992) Physiology of osmotolerance in fungi. Adv Microbial Physiol 33:145–212. https://doi.org/10.1016/S0065-2911(08),60217-9
- Brown AD (1978) Compatible solutes and extreme water stress in eukaryotic microorganisms. Adv Microbial Physiol 17:181–242. https://doi.org/10.1016/S0065-2911(08),60058-2
- Denet E, Vasselon V, Burdin B, Nazaret S, Favre-Bonte S (2018) Survival and growth of Stenotrophomonas maltophilia in free-living amoebae (FLA) and bacterial virulence properties. PLoS One 13(2):e0192308. https://doi.org/10.1371/journal.pone.0192308
- Diver S (2003) Promoting biodynamic practices in Uttaranchal. Technical consultancy report submitted to Farmer-to-Farmer Program (USAID) Winrock International, USA
- Dubey KK, Fulekar MH (2013) Investigation of potential rhizospheric isolate for cypermethrin degradation. 3 Biotech 3:33–43. https://doi.org/10.1007/s13205-012-0067-3
- Estenson K, Hurst GB, Standaert RF, Bible AN, Garcia D, Chourey K, Doktycz MJ, Morrell-Falvey JL (2018) Characterization of Indole-3-acetic Acid biosynthesis and the effects of this phytohormone on the proteome of the plant-associated microbe Pantoea sp. YR343. J Proteome Res 17:1361–1374
- Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195. https://doi.org/10.1104/pp.26.1.192
- Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res 206:131–140. https://doi.org/10.1016/j.micres.2017.08.016
- Gulati A, Sood S, Rahi P, Thakur R, Chauhan S, Chadha IC (2011) Diversity analysis of diazotrophic bacteria associated with the roots of tea (Camellia sinensis (L.) O. Kuntze). J Microbiol Biotechnol 21:545–555. https://doi.org/10.4014/jmb.1012.12022
- Hardy RWF, Holsten RD, Jackson EK (1968) The acetylene-ethylene assay for N2-fixation-laboratory and field evaluation. Plant Physiol 43:118–127. https://doi.org/10.1104/pp.43.8.1185
- Heddi A, Charles H, Khatchadourian C, Bonnot G, Nardon P (1998) Molecular characterization of the principal symbiotic bacteria of the Weevil Sitophilus oryzae: a peculiar G + C content of an endocytobiotic DNA. J Mol Evol 47:52–61. https://doi.org/10.1007/PL00006362
- Hegde SV (2008) Liquid biofertilizers in Indian agriculture. Biofertil Newslett 12:17–22
- Holt JG, Krieg RN, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology. 9. Williams and Wilkins, Baltimore, USA
- Igiehon NO, Babalola OO (2018) Rhizosphere microbiome modulators: contributions of nitrogen fixing bacteria towards sustainable agriculture. Int J Environ Res Public Health 15:574. https://doi.org/10.3390/ijerph15040574
- Imam J, Singh PK, Shukla P (2016) Plant microbe interactions in post genomic era: perspectives and applications. Front Microbiol 7:1488. https://doi.org/10.3389/fmicb.2016.01488
- Imam J, Shukla P, Prasad MN, Variar M (2017) Microbial interactions in plants: perspectives and applications of proteomics. Cur Protein Peptide Sci 18:956–965. https://doi.org/10.2174/1389203718666161122103731
- Karagoz K, Ates F, Karagoz H, Kotan R, Cakmakc R (2012) Characterization of plant growth-promoting traits of bacteria isolated from the rhizosphere of grapevine grown in alkaline and acidic soils. Eur J Soil Biol 50:144–150. https://doi.org/10.1016/j.ejsobi.2012.01.007
- Kaur J, Pandove G, Gangwar M, Brar SK (2018) Development of liquid inoculants: an innovative agronomic practice for sustainable agriculture. J Exp Biol Agric Sci 6:472–481. https://doi.org/10.18006/2018.6(3).472.481
- Khan A, Singh P, Srivastava P (2018) Synthesis, nature and utility of universal iron chelator—siderophore: a review. Microbiol Res 212–213:103–111. https://doi.org/10.1016/j.micres.2017.10.012
- Kumar A, Kumar A, Pratush A (2014) Molecular diversity and functional variability of environmental isolates of Bacillus species. SpringerPlus 3:312. https://doi.org/10.1186/2193-1801-3-312
- Kumar S, Stecher G, Tamura K (2016a) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
- Kumar V, Baweja M, Singh PK, Shukla P (2016b) Recent developments in systems biology and metabolic engineering of plant-microbe interactions. Front Plant Sci 7:1421. https://doi.org/10.3389/fpls.2016.01421
- Lorda G, Balatti A (1996) Designing media I and II. In: Balatti AP, Freire JRJ (eds) Legume inoculants, selection and characterization of strains, production, use and management. Kingraf, Buenos Aires, p 148
- Lowry OH, Rosebrough NJ, Farr AG, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
- Mahanty T, Bhattacharjee S, Goswami M, Bhattacharyya P, Das B, Ghosh A, Tribedi P (2017) Biofertilizers: a potential approach for sustainable agriculture development. Environ Sci Pollut Res 24:3315. https://doi.org/10.1007/s11356-016-8104-0
- Malusa E, Sas-Paszt L, Ciesielska J (2012) Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J 2012:491206. https://doi.org/10.1100/2012/491206
- Marek-Kozaczuk M, Deryto M, Skorupska A (1996) Tn5 insertion mutants of Pseudomonas sp. 267 defective in siderophore production and their effect on clover (Trifolium pratense) nodulated with Rhizobium leguminosarum bv. trifollii. Plant Soil 179:269–274. https://doi.org/10.1007/BF00009337
- Martinez-Hidalgo P, Maymon M, Pule-Meulenberg F, Hirsch AM (2019) Engineering root microbiomes for healthier crops and soils using beneficial, environmentally safe bacteria. Can J Microbiol 65:91–104. https://doi.org/10.1139/cjm-2018-0315
- Mehnaz S, Weselowski B, Lazarovits G (2007) Azospirillum canadense sp. nov., a nitrogen-fixing bacterium isolated from corn rhizosphere. Int J Syst Evol Microbiol 57:620–624. https://doi.org/10.1099/ijs.0.64804-0
- Park M, Kim C, Yang J, Lee H, Shin W, Kim S, Sa T (2005) Isolation and characterization of diazotrophic growth promoting bacteria from rhizosphere of agricultural crops of Korea. Microbiol Res 160:127–133. https://doi.org/10.1016/j.micres.2004.10.003
- Parnell JJ, Berka R, Young HA, Sturino JM, Kang Y, Barnhart DM, DiLeo MV (2016) From the lab to the farm: an industrial perspective of plant beneficial microorganisms. Front Plant Sci 7:1110. https://doi.org/10.3389/fpls.2016.01110
- Ramos PL, Moreira-Filho CA, Trappen SV, Swings J, Vos PD, Barbosa HR, Thompson CC, Vasconcelos ATR, Thompson FL (2011) An MLSA-based online scheme for the rapid identification of Stenotrophomonas isolates. Mem Inst Oswaldo Cruz 106:394–399. https://doi.org/10.1590/S0074-02762011000400003
- Reinhardt EL, Ramos PL, Manfio GP, Barbosa HR, Pavan C, Moreira-Filho CA (2008) Molecular characterization of nitrogen-fixing bacteria isolated from Brazilian agricultural plants at Sao Paulo state. Braz J Microbiol 39:414–422. https://doi.org/10.1590/S1517-83822008000300002
- Ryan RP, Monchy S, Cardinale M, Taghavi S, Crossman L, Avison MB, Berg G, van der Lelie D, Dow JM (2009) The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat Rev 7:514–525. https://doi.org/10.1038/nrmicro2163
- Saraf M, Thakker A, Patel BV (2008) Biocontrol activity of different species of Pseudomonas against phytopathogenic fungi in vivo and in vitro conditions. Int J Biotechnol Biochem 4:223–232
- Saribay GF (2003) Growth and nitrogen fixation dynamics of Azotobacter chroococcum in nitrogen-free and OMW contaminating medium. M.Sc. Thesis, The Graduate School of Natural and Applied Sciences of the Middle East Technical University
- Schwyn B, Neilands JB (1987) Universal chemical assay for detection and determination of siderophore. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
- Singh RP, Jha PN (2017) The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front Microbiol 8:1945. https://doi.org/10.3389/fmicb.2017.01945
- Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448. https://doi.org/10.1111/j.1574-6976.2007.00072.x
- Sui J, Ji C, Wang X, Liu Z, Sa R, Hu Y, Wang C, Li Q, Liu X (2019) A plant-growth promoting bacterium alters the microbial community of continuous cropping poplar trees rhizosphere. J Appl Microbiol. https://doi.org/10.1111/jam.14194
- Sunder S, Singh AJ, Gill S, Singh B (1996) Regulation of intracellular level of Na+, K+ and glycerol in Saccharomyces cerevisiae under osmotic stress. Mol Cell Biochem 158:121–124. https://doi.org/10.1007/BF00225837
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighing, position-specific gap penalties and weight matrix choice. Nucleic Acid Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
- Tkacz A, Poole P (2015) Role of root microbiota in plant productivity. J Exp Bot 66:2167–2175. https://doi.org/10.1093/jxb/erv157
- Tsavkelova EA, Cherdyntseva TA, Botina SG, Netrusov AI (2007) Bacteria associated with orchid roots and microbial production of auxin. Microbiol Res 162:69–76. https://doi.org/10.1016/j.micres.2006.07.014
- Tyagi S, Mulla SI, Lee KJ, Chae JC, Shukla P (2018) VOCs-mediated hormonal signaling and crosstalk with plant growth promoting microbes. Crit Rev Biotechnol 38:1277–1296. https://doi.org/10.1080/07388551.2018.1472551
- Vejan P, Abdullah R, Khadiran T, Ismail S, Boyce AN (2018) Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21:573. https://doi.org/10.3390/molecules21050573
- Venieraki A, Dimou M, Pergalis P, Kefalogianni I, Chatzipavlidis I, Katinakis P (2011) The genetic diversity of culturable nitrogen-fixing bacteria in the rhizosphere of wheat. Microb Ecol 61:277–285. https://doi.org/10.1007/s00248-010-9747-x
- Verma M, Mishra J, Arora NK (2019) Plant growth-promoting rhizobacteria: diversity and applications. In: Sobti RC, Arora NK, Kothari R (eds) Environmental biotechnology: for sustainable future. Springer, Singapore, pp 129–173
- Winkelmann G (1991) Specificity of iron transport in bacteria and fungi. In: Winkelmann G (ed) Handbook of microbial iron chelates. CRC Press, Boca Raton, pp 65–105
Author Information
Department of Microbiology, College of Basic Sciences, CSK Himachal Pradesh Agricultural University, Palampur, India