In vitro propagation of Costa Rican guava (Psidium friedrichsthalianum (O. Berg) Nied.) plantlets from seedlings in the temporary immersion system RITA®, rooting and acclimatization
*Article not assigned to an issue yet
Sánchez-Calvo Laura, Avendaño Andrea, Orozco-Ortiz Cristofer, Morales Alejandra, Vargas-Solórzano Isaac, Montoya Andrés, Araya-Valverde Emanuel
Research Articles | Published: 26 March, 2025
First Page: 0
Last Page: 0
Views: 651
Keywords: BAP, IBA, Temporary immersion systems, RITA®, Rooting
Abstract
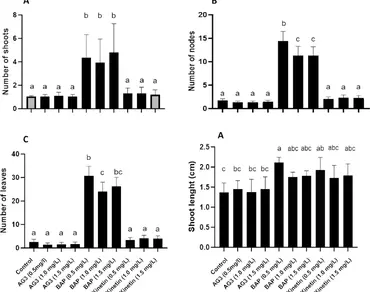
Fruits of Costa Rican guava (Psidium friedrichsthalianum (O. Berg) Nied) contain phenolic compounds and offer the possibility of creating novel functional foods with benefits for human health. In vitro culture, a propagation technique currently scarce for this species, can be developed for the propagation of genotypes with desirable fruit and agronomic traits. Woody Plant Medium supplemented with the growth regulators 6-benzylaminopurine, kinetin and gibberellic acid at concentrations of 0.5, 1.0 and 1.5 mg/L was used. Microcutting resulted in a more favorable response to BAP for variables such as the number of shoots (NS), number of nodes (NN) and number of leaves (NL). The mean NS was 4.80 when 1.5 mg/L BAP was used, whereas the optimal response for NN (14.73), NL (30.73), and shoot length (SL) (2.2 cm) was 0.5 mg/L BAP. WPM was subsequently supplemented with 0.5 mg/L BAP for multiplication in RITA®. The number of shoots (6.33 ± 2.31) and multiplication coefficient (5.33 ± 2.31) were greater than those of the semisolid media, with values of 4.30 ± 1.32 and 3.30 ± 1.32, respectively. For the rooting phase, the greatest response to the number of roots (3.75 ± 2.79), root length (2.46 ± 1.61 cm) and plant height (7.00 ± 0.63 cm) was associated with 0.3 mg/L indol-3-butyric acid. The survival rate of the rooted plantlets in the greenhouse was greater than 90% when the plants were exposed to IBA. This work provides new knowledge to the scarce literature on in vitro culture of P. friedrichsthalianum, particularly on the use of RITA®, which is described for the first time. New contributions to rooting and acclimatization for this species are comprehensively detailed.
References
Ali N, Mulwa RMS, Norton MA, Skirvin RM (2003) Micropropagation of guava (Psidium guajava L.). J Hortic Sci Biotechnol 78:739–741. https://doi.org/10.1080/14620316.2003.11511692
Ali N, Mulwa RMS, Norton MA, Skirvin RM (2007) Radical disinfestation protocol eliminates in vitro contamination in guava (Psidium guajava L.) seeds. Plant Cell Tissue Organ Cult 91:295–298. https://doi.org/10.1007/s11240-007-9283-7
Andújar I, González N, García-Ramos JC, Bogdanchikova N, Pestryakov A, Escalona M, Concepción O (2020) Argovit™ silver nanoparticles reduce contamination levels and improve morphological growth in the in vitro culture of Psidium friedrichsthalianum (O. Berg) Nied. SN Appl Sci 2:1–9. https://doi.org/10.1007/s42452-020-03948-9
Baraona M, Rivera G (1995) Desarrollo del jocote (Spondias purpurea L.) y del cas (Psidium friedrichstlzalianum (Berg.) Niedz) en el bosque húmedo premontano de Costa Rica. Agron Mesoam 6:23. https://doi.org/10.15517/am.v6i0.24804
Chinchilla FG, Villegas E, Molina A, Arias C (2016) Composition, chemical fingerprinting and antimicrobial assessment of costa rican cultivated guavas (Psidium friedrichsthalianum (O. Berg) Nied. and Psidium guajava L.) essential oils from leaves and fruits. Nat Prod Chem Res 4:4–6. https://doi.org/10.4172/2329-6836.1000236
Cuadrado-Silva CT, Pozo-Bayón MÁ, Osorio C (2017) Targeted metabolomic analysis of polyphenols with antioxidant activity in sour guava (Psidium friedrichsthalianum Nied.) fruit. Molecules 22:1–10. https://doi.org/10.3390/molecules22010011
de Oliveira Gentil DF, da Nascimento Ferreira SA, Rebouças ER (2018) Germination of psidium friedrichsthalianum (O. Berg) nied. seeds under different temperature and storage conditions. J Seed Sci 40:246–252. https://doi.org/10.1590/2317-1545v40n3179617
Domínguez-Perales LA, Domínguez-Álvarez JL, Cruz-Izquierdo S, Santacruz-Varela A, Barrientos-Priego A, Padilla-Ramírez JS, Gutiérrez-Espinosa MA (2016) Propagación in vitro de selecciones de Guayabo (Psidium guajava L.). Rev Fitotec Mex 39:285–295. https://doi.org/10.35196/rfm.2016.3.285-295
dos Santos MAC, do Rêgo MM, de Queiróz MA et al (2020) In vitro growth performance of Psidium guajava and P. guineense plantlets as affected by culture medium formulations. Vegetos 33:435–445. https://doi.org/10.1007/s42535-020-00125-6
Durán-Castañeda AC, Cardenas-Castro AP, Pérez-Jiménez J, Pérez-Carvajal AM, Sánchez-Burgos JA, Mateos R, Sáyago-Ayerdi SG (2023) Bioaccessibility of phenolic compounds in Psidium guajava L. varieties and P. friedrichsthalianum Nied. after gastrointestinal digestion. Food Chem 400:134046. https://doi.org/10.1016/j.foodchem.2022.134046
Elezaby AA, Allatif AMA (2017) In vitro propagation of guava (Psidium guajava L.): effect of antioxidant, nutrient media and growth regulators. J Hortic Sci Ornam Plants 9:144–149
Etienne H, Berthouly M (2002) Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult 69:215–231. https://doi.org/10.1023/A:1015668610465
Flores G, Dastmalchi K, Wu SB, Whalen K, Dabo AJ, Reynertson KA, Foronjy RF, D´Armiento JM, Kenelly EK (2013) Phenolic-rich extract from the Costa Rican guava (Psidium friedrichsthalianum) pulp with antioxidant and anti-inflammatory activity. Potential for COPD therapy. Food Chem 141:889–895. https://doi.org/10.1016/j.foodchem.2013.03.025
Freitas VM, Correa VR, Motta FC, Sousa MG, Gomes ACMM, Carneiro MDG, Silva DB, Mattos JK, Nicole M, Carneiro RMDG (2014) Resistant accessions of wild Psidium spp. to Meloidogyne enterolobii and histological characterization of resistance. Plant Pathol 63:738–746. https://doi.org/10.1111/ppa.12149
Georgiev V, Schumann A, Pavlov A, Bley T (2014) Temporary immersion systems in plant biotechnology. Eng Life Sci 14:607–621. https://doi.org/10.1002/elsc.201300166
González-Ball R, Bermúdez-Rojas T, Romero-Vargas M, Ceuterick M (2022) Medicinal plants cultivated in urban home gardens in Heredia, Costa Rica. J Ethnobiol Ethnomed 18:1–19. https://doi.org/10.1186/s13002-022-00505-z
Grattapaglia D, Vaillancourt RE, Shepherd M, Thumma BR, Foley W, Külheim C, Potts MP, Myburg AA (2012) Progress in Myrtaceae genetics and genomics: eucalyptus as the pivotal genus. Tree Genet Genomes 8:463–508. https://doi.org/10.1007/s11295-012-0491-x
Jaiswal VS, Amin MN (1987) In Vitro propagation of guava from shoot cultures of mature trees. J Plant Physiol 130:7–12. https://doi.org/10.1016/S0176-1617(87)80296-1
Kala S, Sharma S, Kajla S, Mir H (2017) In vitro multiplication of guava rootstocks: Psidium Guajava cv. Lucknow-49 and Psidium Friedrichsthalianum (Chinese Guava). Indian J Ecol 44:488–493
Lim TK (2012) Psidium friedrichsthalianum. Edible medicinal and non-medicinal plants: volume 3, fruits. Springer, Dordrecht Heidelberg, London, pp 681–683
Liu X, Yang G (2011) Clonal propagation of guava (Psidium guajava L) on nodal explants of mature elite cultivar. Int J Plant Biol 2:7–10. https://doi.org/10.4081/pb.2011.e2
Lloyd G, McCown B (1980) Commercially-Feasible micropropagation of mountain Laurel, Kalmia latifolia, by use of shoot-tip culture. Proc Int Plant Propagator´s Soc 30:421–427
Mani A, Mishra R, Thomas G (2011) Elucidation of diversity among psidium species using morphological and SPAR methods. J Phytol 3:53–61
Mirzabe AM, Hajiahmad A, Fadavi A, Rafiee S (2022) Temporary immersion systems (TISs): a comprehensive review. J Biotech 357:56–83. https://doi.org/10.1016/j.jbiotec.2022.08.003
Mitra SK, Irenaeus TKS, Gurung MR, Pathak PK (2012) Taxonomy and importance of myrtaceae. Acta Hortic 959:23–34. https://doi.org/10.17660/ActaHortic.2012.959.2
Mohamed-Yasseen Y, Barringer SA, Schnell RJ, Splittstoesser WE (1995) In vitro shoot proliferation and propagation of guava (Psidium guajava L.) from germinated seedlings. Plant Cell Rep 14:525–528. https://doi.org/10.1007/BF00232788
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Rai MK, Jaiswal VS, Jaiswal U (2009) Shoot Multiplication and plant regeneration of Guava (Psidium guajava L.) from nodal explants of invitro raised plantlets. J Fruit Ornam Plant Res 17:29–38
Rebouças ER, Gentil DFO, Ferreira SADN (2008) Caracterização física de frutos e sementes de goiaba-da- costa-rica, produzidos em Manaus. Amazonas Rev Bras Frutic 30:546–548. https://doi.org/10.1590/S0100-29452008000200048
Rico S, Garrido J, Sánchez C, Ferreiro-Vera C, Codesido V, Vidal N (2022) A temporary immersion system to improve cannabis sativa micropropagation. Front Plant Sci 13:895971. https://doi.org/10.3389/fpls.2022.895971
Rojas-Garbanzo C (2018) Psidium Fruits : Endemic Fruits of Latin America with a Wide variety of phytochemicals. Ann Nutr Food Sci 2:1016
Rojas-Garbanzo C, Zimmermann BF, Schulze-Kaysers N, Schieber A (2017) Characterization of phenolic and other polar compounds in peel and flesh of pink guava (Psidium guajava L. cv. ‘Criolla’) by ultra-high performance liquid chromatography with diode array and mass spectrometric detection. Food Res Int 100:445–453. https://doi.org/10.1016/j.foodres.2016.12.004
Rojas-Garbanzo C, Winter J, Montero ML, Zimmerman BF (2019) Characterization of phytochemicals in Costa Rican guava (Psidium friedrichsthalianum -Nied.) fruit and stability of main compounds during juice processing - (U)HPLC-DAD-ESI-TQD-MSn. J Food Compos Anal 75:26–42. https://doi.org/10.1016/j.jfca.2018.09.012
Shah ST, Zamir R, Ahmad J et al (2008) In vitro regeneration of plantlets from seedlings explants of guava (Psidium guajava L.) Cv. Safeda Pakistan J Bot 40:1195–1200
Stefanello MEA, Pascolan A, Salvador M (2011) Essential oils from neotropical myrtaceae: chemical diversity and biological properties. Chem Biodivers 8:73–94. https://doi.org/10.1002/cbdv.201000098
Tzatzani TT, Michail I, Boutsika A et al (2023) Micropropagation of guava (Psidium guajava) seedlings, a plant with interest in cool subtropics, using an innovative BB culture medium. Biotechnol Biotechnol Equip 37:139–150. https://doi.org/10.1080/13102818.2022.2159524
Valdiani A, Hansen OK, Nielsen UB, Johannsen VK, Shariat M, Georgiev MI, Abiri R (2018) Bioreactor-based advances in plant tissue and cell culture: challenges and prospects. Crit Rev Biotechnol 39:20–34. https://doi.org/10.1080/07388551.2018.1489778
Author Information
Cátedra de Investigación, Extensión y Tecnología Agropecuaria, Universidad Estatal a Distancia (UNED), San José, Costa Rica