In vitro induction and plant regeneration in potato (Solanum tuberosum L.) cv. Kufri Sangam
*Article not assigned to an issue yet
Batta Sudha, Thakur Ajay Kumar, Singh Rajender, Singh Sakshi, Thakur Pitambri, Gupta Reena
Research Articles | Published: 05 March, 2025
First Page: 0
Last Page: 0
Views: 992
Keywords: Solanaceae, Micropropagation, Callus culture, Tissue culture, IAA
Abstract
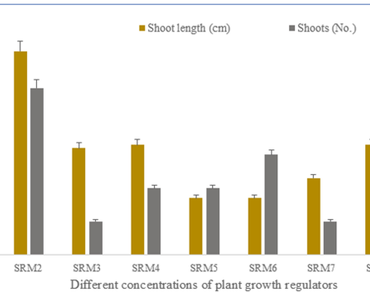
Potato (Solanum tuberosum L.) is a perennial food crop that helps to ensure global food and nutrition security. This study developed an in vitro callus and shoot induction protocol for the potato cultivar Kufri Sangam and assessed the frequency of callus and shoot regeneration. This study found that supplementing the Murashige and Skoog (MS) medium with 0.5 mgL−1 6-Benzylaminopurine (BAP) and 5 mgL−1 1-Naphthaleneacetic acid (NAA) resulted in 100% callus induction whereas 2 mgL−1 thiadiazuron (TDZ) and 0.25 mgL−1 1-Naphthaleneacetic acid (NAA) was most effective for shoot induction. For shoot proliferation, MS media supplemented with zeatin riboside (1 mgL−1), NAA (0.5 mgL−1), and GA3 (5 mgL−1), resulted in 73.33% shoot regeneration. The effect of varying indole butyric acid (IBA) on root induction showed the best results when MS medium was supplemented with 1.0 mgL−1 IBA. By allowing the micropropagation of an isogenic population in a highly heterozygous potato plant, well-optimized culture conditions and the right combinations of growth regulators guarantee an effective regeneration technique for a particular potato cultivar and reduce the likelihood of somaclonal variants. The application of biotechnological developments in gene transfer and ploidy level maintenance in the potato cultivar Kufri Sangam may benefit from the deployment of this innovative approach.
References
Abeuova LS, Kali BR, Rakhimzhanova AO, Bekkuzhina SS, Manabayeva SA (2020) High frequency direct shoot regeneration from Kazakh commercial potato cultivars. Peer J 8:e9447. https://doi.org/10.7717/peerj.9447
Abu Zeid IM, Soliman HIA, Metwali EMR (2022) In vitro evaluation of some high yield potato (Solanum tuberosum L.) cultivars under imposition of salinity at the cellular and organ levels. Saudi J Biol Sci 29(4):2541–2551
Adly WMRM, Niedbała G, EL-Denary ME, Mohamed MA, Piekutowska M, Wojciechowski T, Abd El-Salam E-ST, Fouad AS (2023) Somaclonal variation for genetic improvement of starch accumulation in potato (Solanum tuberosum) tubers. Plants 12(2):232. https://doi.org/10.3390/plants12020232
Alexopoulos A, Petropoulos S (2021) Tissue culture of potato for seed production. The potato crop. management, production and food security Nova science publishers, New York, pp 61–90
Aprilia M, Setiari N, Nurchayati Y (2022) Callus Development from Potato (Solanum tuberosum L.) Stem at Various Concentrations of Benzylaminopurine. Biosaintifika: J Biol Biol Ed 14(2):219–225
Dar SA, Nawchoo IA, Tyub S, Kamili AN (2021) Effect of plant growth regulators on in vitro induction and maintenance of callus from leaf and root explants of Atropa acuminata Royal ex Lindl. Biotech Rep (Amst) 14(32):e00688. https://doi.org/10.1016/j.btre.2021.e00688
de Morais TP, Asmar SA, de Jesus Silva HF, Luz JMQ, de Melo B (2018) Application of tissue culture techniques in potato. Biosci J 34(4):952–969
Dessoky EDS, Attia AO, Ismail IA, El-Hallous EI (2016) In vitro propagation of potato under different hormonal combinations. Int J of Adv Res 4:684–689
Kaur A, Reddy M, Kumar A (2017) Efficient, one step and cultivar independent shoot organogenesis of potato. Phys Mol Bio Plants 23:461–469. https://doi.org/10.1007/s12298-017-0418-y
Kondhare KR, Patil AB, Giri AP (2021) Auxin: An emerging regulator of tuber and storage root development. Plant Sci 306:110854. https://doi.org/10.1016/j.plantsci.2021.110854
Kumlay AM, Ercisli S (2015) Callus induction, shoot proliferation and root regeneration of potato (Solanum tuberosum L.) stem node and leaf explants under long-day conditions. Biotech Biotechnol Equip 29(6):1075–1084
Liu Y, Andersson M, Granell A, Cardi T, Hofvander P, Nicolia A (2022) Establishment of DNA-free genome editing and protoplast regeneration method in cultivated tomato (Solanum lycopersicum). Plant Cell Rep 41:1843–1852. https://doi.org/10.1007/s00299-022-02893-8
Luthra SK, Kumar V (2024) Potato genetic resources and their utilization in India. Indian J Plant Genet Resour 37(1):1–19
Mali S, Dutta M, Zinta G (2023) Genome editing advancements in potato (Solanum tuberosum L.): operational challenges and solutions. J Plant Biochem Biotechnol 32:730–742. https://doi.org/10.1007/s13562-022-00812-2
Munthali C, Kinoshita R, Onishi K, Rakotondrafara A, Mikami K, Koike M, Tani M, Palta J, Aiuchi D (2022) A model nutrition control system in potato tissue culture and its influence on plant elemental composition. Plants 11(20):2718. https://doi.org/10.3390/plants11202718
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. J Plant Physiol 1:473–479
Ohnuma M, Teramura H, Shimada H (2020) A simple method to establish an efficient medium suitable for potato regeneration. Plant Biotechnol 37(1):25–30
Rani S, Deepti Kumari A, Sharma VK (2021) Effect of explant and phytohormone on in vitro regeneration of Solanum indicum, an important medicinal weed. J Pharm Phyto 10(1):1070–1075
Setiaji A, Annisa RR, Rumiyati R, Semi AE (2020) Induction and growth kinetics callus of tomato (Solanum lycopersicum). Biosaintifika: J Biol Biol Edu 12(1):35–41
Shekhawat M, Manokari M (2016) Impact of auxins on vegetative propagation through stem cuttings of Couroupita guianensis Aub: a conservation approach. Scientifica. https://doi.org/10.1155/2016/6587571
Shukla SR, Zala HN, Solanki SD, Ant HM (2023) Optimizing microtubers production for sustainable potato cultivation in Gujarat. India Biol Life Sci Forum 27(1):2. https://doi.org/10.3390/IECAG2023-15488
Teo CJ, Takahashi K, Shimizu K, Shimamoto K, Taoka K (2017) Potato tuber induction is regulated by interactions between components of a tuberigen complex. Plant Cell Physiol 58:365–374
The Potato Genome Sequencing Consortium (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195. https://doi.org/10.1038/nature10158
Tripathi L, Ntui VO, Tripathi JN (2022) Control of bacterial diseases of banana using CRISPR/Cas-Based gene editing. Int J Mol Sci 23:3619. https://doi.org/10.3390/ijms23073619
Velda AC, Rodriguez SAG, Descalsota MLV, Damasco OP (2023) Thidiazuron-mediated and genotype-independent regeneration system for tomato (Solanum lycopersicum L.). J Appl Biol Biotech 11(4):128–134
Xu X, Pan S, Cheng S, Zhang B, Mu D, Ni P, Zhang G, Yang S, Li R, Wang J, Orjeda G, Guzman F, Torres M, Lozano R, Ponce O, Martinez D, De la Cruz G, Chakrabarti SK, Patil VU et al (2011) Genome sequence and analysis of the tuber crop potato. Nature 475(7355):189–195
Yasumoto S, Umemoto N, Lee HJ, Nakayasu M, Sawai S, Sakuma T, Yamamoto T, Mizutani M, Saito K, Muranaka T (2019) Efficient genome engineering using Platinum TALEN in potato. Plant Biotechnol 36:167–173
Ying HS, Xian SZ (2014) The hormonal control of regeneration in plants. Curr Top Dev Biol 108:35–36
Yousif S (2015) Original research article effect of different medium on callus induction and regeneration in potato cultivars. Int J Curr Microbiol App Sci 4(5):856–865
Author Information
Department of Biotechnology, Himachal Pradesh University, Shimla, India