In-vitro antibacterial activities of fermented and unfermented Parkia biglobosa seeds against selected entero-pathogens
Ilesanmi Victor O., Adegbehingbe Kehinde T., Oyeniyi Daniel O., Oke Oluwatosin G., Dada Adebowale D.
Research Articles | Published: 30 August, 2023
First Page: 566
Last Page: 577
Views: 3316
Keywords: Parkia biglobosa , Fermentation, Entero-pathogens, Antibacterial, Proximate, And antioxidants
Abstract
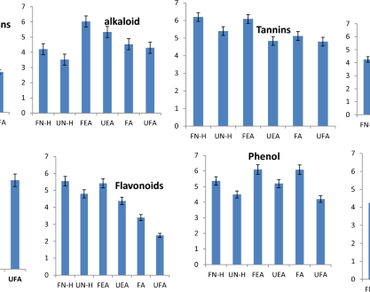
The study examined the antibacterial effects of both fermented and unfermented ethanolic extracts of Parkia biglobosa seeds against selected entero-pathogenic bacteria. The antibacterial activity was evaluated using agar well diffusion to determine the effect of the extracts against selected entero-pathogenic bacteria; Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus haemoliticus, Escherichia coli, Citrobacter youngae, Klebsiella oxytoca and Acinetobacter haemolyticus. Antioxidant activities of the extracts were quantified by measuring its total flavonoid and total phenol contents, 1,1-diphenyl-2-picrylhydrazyl, 2,2ʹ-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid and ferric reducing antioxidant power. Phytochemical screening and Gas Chromatography–Mass Spectrometry were carried out on both samples using standard methods. The sensitivity test revealed that E. coli, S. aureus, C. youngae, A. haemolyticus and K. oxytoca were more susceptible (25.0 mm, 24.0 mm, 18.0 mm, 27.0 mm, and 17.0 mm) at 100 mg/ml to the crude ethanolic extract of fermented seeds, while S. aureus, E. coli, and A. haemolyticus were more susceptible to the unfermented extract. The seed extracts of fermented and unfermented P. biglobosa were found to be high in phytochemicals such as alkaloids, flavonoids, cardiac, steroids, glycosides, saponins, and tannins. Results showed that calcium, magnesium, sodium, potassium, zinc, iron, phosphorus, manganese, and copper were present in fermented and unfermented samples. The findings indicated that P. biglobosa seeds had considerably high and dose-dependent DPPH radical scavenging and ferric-reducing properties comparable with respective standards. The various constituents of P. biglobosa extracts enhanced its antibacterial activities. This study concluded that P. biglobosa can be a potential source of bioactive compounds and antioxidants.
References
Abdel-Rahman MA, Yukihiro T, Kenji S (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotech Adv 31(2013):877–902
Abioye EO, Akinpelu DA, Aiyegoro OA (2013) Preliminary phytochemical screening and antibacterial properties of crude stem bark extracts and fractions of Parkia biglobosa (Jacq.). Molecules 18(7):8459–8499
Achi OK (2005) The upgrading of traditional fermented foods through biotechnology. Afr J Biotech 4:375–380
Adetutu A, Morgan W, Corcoran O (2011) Ethnopharmacological survey and in vitro evaluation of wound-healing plants used in South-western Nigeria. J Ethnopharm 137(1):50–56
Ajaiyeoba EO (2002) Phytochemical and antibacterial properties of Parkia biglobosa and Parkia bicolor leaf extracts. Afr J Biomed 5:125–129
AOAC (2005) Official method of analysis, 18th ed. Association of Official Analytical Chemists, Washington D.C.
AOAC (2016) Official methods of analysis, 20th ed. Association of Official Analytical Chemist, Washington D.C.
Ayo-Lawal RA, Osoniyi O, Famurewa AJ, Lawal OA (2014) Evaluation of antioxidant and hypolipidaemic effects of fermented Parkia biglobosa (Jacq) seeds in tyloxapol-induced hyperlipidaemic rats. Afr J Food Sci 8(5):225–232
Bao JY, Cai M, Sun G, Wang C (2005) Anthocyanins, flavonoid and free radical scavenging activity of thines Baybery (Myrial rubia) extracts and their colour properties and stability. J Agric Food Chem 53:2327–2332
Cao G, Sofic E (2006) Priorities in the use of common hydrophilic and lipophilic antioxidant assays in health food evaluation. J Agric Food Chem 54(3):889–896. https://doi.org/10.1021/jf052308+
Clinical, Laboratory Standards Institute (CLSI) (2016) Performance standards for antimicrobial susceptibility testing. In: Twenty-sixth informational supplement. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne
Daramola B (2015) Effects of extraction solvent, morphological parts and ripening stage on antioxidative activity of Solanum anguivi fruit. Int Food Res J 22:2
Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4:685–688
Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109:69–75
Gautam RK, Dixit PK, Mittal S (2013) Herbal sources of antidepressant potential: a review. Int J Pharm Sci Rev Res 18:86–91
GraphPad Prism (2018) GraphPad Prism 8th edition. GraphPad Software Inc
Gutierrez J, Barry-Ryan C, Bourke P (2008) The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int J Food Microb 124:91–97
Harborne JB (1998) A guide to modern technique of plant analysis, 3rd edn. Chapman and Hall, London, p 285
Iheke E, Oshodi A, Omoboye A, Ogunlalu O (2017) Effect of fermentation on the physicochemical properties and nutritionally valuable minerals of locust bean (Parkia biglobosa). Am J Food Tech 12(6):379–384
Komolafe K, Olaleye TM, Omotuyi OI (2014) In vitro antioxidant activity and effect of Parkia biglobosa bark extract on mitochondrial redox status. J Acup Merid Stud 7(4):202–210
Kumar RS, Rajkapoor B, Perumal P (2012) Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac J Trop Biomed 2(4):256–261. https://doi.org/10.1016/S2221-1691(12)60019-7
McGaw LJ, Jäger AK, Van Staden J (2002) Antibacterial effects of fatty acids and related compounds from plants. South Afri J Bot 68:417–423
Mente A, O’Donnell M, Yusuf S (2021) Sodium intake and health: what should we recommend based on the current evidence? Nutrients 13(9):3232. https://doi.org/10.3390/nu13093232
Millogo-Kone H, Guissou I, Nacoulma O, Traore AS (2007) Antimicrobial effects of the stem bark extracts of Parkia biglobosa (Jacq.) Benth. on Shigellae. Afr J Tradition Complement Alternat Med 4(4):392–396
Muhammad SA, Syeda AB (2017) Natural antimicrobials, their sources and food safety. Food Add. https://doi.org/10.5772/intechopen.70197
Muhammed M, Yusuf AA, Odey BO, Alawode AR, Adegbola GA, Agboola RA (2021) A systematic review of domestication, ethnopharmacological use, phytochemistry, nutritional composition, and biological activities of Parkia biglobosa. BIOMED Nat Appl Sci 1(1):01–12
Nitiema-Yefanova S, Dossa CP, Gbohaïda V, Kanfon RE, Mossi I, Beakou BH (2020) Fermented Parkia biglobosa seeds as a nitrogen source supplementation for bioethanol production from cashew apple juice. Int J Biol Chem Sci 14(9):3441–3454
Ogbole OO, Akinmoladun AC (2020) Phytochemical analysis and antimicrobial activity of Parkia biglobosa seed oil. J Pharm Res Int 32(20):109–115. https://doi.org/10.9734/jpri/2020/v32i2030734
Okwunodulu FU, Friday C, Okwunodulu IN, Chukwudi VI (2021) Comparative study on the phytochemicals, proximates, vitamins and mineral elements compositions of unfermented and fermented seeds of Parkia biglobosa. Biosci Biotechnol J 2(1):7–10
Oladipupo BB, Adebayo-Tayo BC, Sodipo OA (2013) Phytochemical and GC-MS analysis of ethanol and acetone extracts of Parkia biglobosa leaves, stem bark and root bark. IOSR J Appl Chem 4(3):12–17
Oluwaniyi O, Bazambo IO (2016) Nutritional and amino acid analysis of raw, partially fermented and completely fermented locust bean (Parkia biglobosa) Seeds. Afr J Food Agric Dev 16(2):10867–10875
Omafuvbe BO, Falade OS, Osuntogun BA, Adewusi SR (2004) Chemical and biochemical changes in African locust bean (Parkia biglobosa) and melon (Citrullus vulgaris) seeds during fermentation to condiments. Pak J Nutr 3(3):140–145
Osuntokun O, Akele E, Paul D (2020) Evaluation of fermented Parkia biglobosa (African locust bean) and Bombax evaluation of fermented Parkia biglobosa (African locust bean) and Bombax glabra (Malabar chest nut). J Bacteriol Mycol 7:1148
Oyeleke SB, Dauda BE, Boye OA (2008) Antibacterial activity of Ficus capensis. Afr J Biotech 7(10):1414–1417
Parekh J, Chanda SV (2007) In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turkey J Biol 31:53–58
Plessas S (2022) The rendering of traditional fermented foods in human diet: distribution of health benefits and nutritional benefits. Fermentation 8(12):751
Pulido R, Bravo L, Saura-Calixto F (2002) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48:3396–3402
Puntel RL, Nogueira CW, Rocha JB (2005) Krebs cycle intermediates modulate Thiobarbituric Acid Reactive Species (TBARS) production in rat brain in vitro. Neurochem Res 30:225–235
Re R, Pellegrin N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improve ABTS radication decolourization assay. Free Rad Biol Med 26:1231–1237
Sharma R, Garg P, Kumar P, Bhatia SK, Kulshrestha S (2020) Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 6(4):106–109
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Cioalteau reagents. Methods Enzymo 299:152–178
Soetan KO, Olaiya CO, Oyewole OE (2010) The importance of mineral elements for humans, domestic animals, and plants: a review. Afr J Food Sci 4(5):200–222
Soetan KO, Akinrinde AS, Adisa SB (2014) Comparative studies on the proximate composition, mineral and antinutritional factors in the seeds and leaves of African locust bean (Parkia biglobosa). Ann Food Sci Technol 15(1):70
Sun JS, Tsuang YH, Chen IJ, Huang WC, Hang YS, Lu FJ (1998) An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns J Int Soc Burn Injur 24(3):225–231. https://doi.org/10.1016/s0305-4179(97)00115-0
Uche-Ikonne CO, Ebere CO, Nwachoko N (2019) Essential oil extraction and characterization of Parkia biglobosa. J Pharm Res Int 29(6):1–13. https://doi.org/10.9734/jpri/2019/v29i630259
Author Information
Department of Microbiology, Adekunle Ajasin University, Akungba Akoko, Nigeria