High speed regeneration via somatic embryogenesis in elite Indian banana cv. Somrani monthan (ABB)
Research Article | Published: 20 February, 2019
First Page: 39
Last Page: 47
Views: 3987
Keywords: Banana, Embryogenic calli, Embyrogenic cell suspension, Multiple shoot clump, Somatic embryogenesis
Abstract
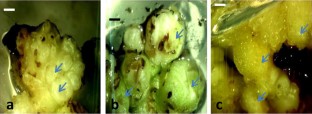
An efficient and rapid regeneration system is indispensable for germplasm conservation and valuable trait incorporation through genetic engineering. The present study was aimed to investigate regeneration potential of Musa sp. cv. Somrani monthan (ABB) via somatic embryogenesis. Meristematic shoot tips were used as explant and cultured on MS medium supplemented with 6-BAP (50 and 100 µM) and IAA (1 µM) for scalp (cauliflower like compact buds) induction. The highest percentage (~ 60%) of homogenous differentiation of shoot tip explants into scalp was observed in MS medium supplemented with 100 µM of 6-BAP and 1 µM of IAA after 6 months of culture. The scalps were sub-cultured on MS medium supplemented with different compositions of 2, 4-D (1, 2, 3, 4, 5 and 10 µM) and zeatin (1 and 2 µM) for embryogenic calli induction. The highest frequency (97%) of embryogenic calli induction was recorded in MS medium supplemented with 5 µM 2, 4-D and 1 µM zeatin after 6 weeks of culture. The histological and morphological studies suggest that, callus possessed high competence for embryogenesis and could be induced to form somatic embryos. Embryogenic cell suspension (ECS) was successfully established by culturing these embryonic calli in liquid medium. About 70% of the mature somatic embryos germinated and converted to plantlets with a regeneration capacity of 8.5 × 103. After the gradual acclimatization and hardening, the plants were transferred to nursery with 82% of survival rate. Here we report a successful regeneration system for an elite Indian Musa cv. Somrani monthan via somatic embryogenesis. The embryogenic callus and ECS are ideal explants for mass clonal propagation, germplasm conservation and genetic transformation for future research.
References
- Ali KS, ELHassan AA, Ehiweris SO, Maki HE (2013) Embryogenesis and plantlet regeneration via immature male flower culture of banana (Musa spp.)cv. Grand Nain. J Prod Ind 3:48–52
- Becker DK, Dugdale B, Smith MK, Harding RM, Dale JL (2000) Genetic transformation of Cavendish banana (Musaspp. AAA group) cv. Grand Nain via micro-projectile bombardment. Plant Cell Rep 19:229–234
- Boissot N, Valdez M, Guiderdoni E (1990) Plant regeneration from leaf and seed-derived calli and suspension cultures of the African perennial wild rice, Oryza longistaminata. Plant Cell Rep 9:447–450
- Cote FX, Domergne R, Monmarson S, Schwendiman J, Teisson C, Escalant JV (1996) Embryogenic cell suspensions from male flower of Musa AAA cv. Grand Nain. Physiol Plant 97:285–290
- Dhed’a D, Dumortier F, Panis B, Vuylsteke D, De Langhe E (1991) Plant regeneration in cell suspension cultures of the cooking banana cv. “Bluggoe” (Musa spp. ABB group). Fruits 46:125–135
- Escalant JV, Teisson C (1989) Somatic embryogenesis plants from immature zygotic embryos of species Musa acuminata and Musa balbisiana. Plant Cell Rep 7:665–668
- Escalant JV, Teisson C, Cote F (1994) Amplified somatic Embryogenesis From male flowers of triploid banana and plantain cultivars (Musa spp.). In Vitro Cell Dev Biol Plant 30:181–186
- Ferdous MH, Billah MA, Mehraj H, Taufique T, UddinAFM Jamal (2015) BAP and IBA pulsing for in vitro multiplication of banana cultivars through shoot-tip culture. J Biosci Agric Res 03:87–95
- Ganapathi TR, Suprassna P, Bapat VA, Kulkarni VM, Rao PS (1999) Somatic embryogenesis and plant regeneration from male flower buds in banana. Curr Sci 76:1228–1231
- Ganapathi TR, Higgs NS, Ballint-Kurti PJ, Arntzen CJ, May GD, Van Eck JM (2001) Agrobacterium mediated transformation of embryogenic cell suspension of the banana cultivar Rasthali (AAB). Plant Cell Rep 29:157–162
- Grapin A, Schwendiman J, Teisson C (1996) Somatic embryogenesis in plantain bananas. Vitro Cell Dev Biol Plant 32:66–71
- Grapin A, Ortiz JL, Lescot T, Ferriere N, Cote FX (2000) Recovery and regeneration of embryogenic cultures from female flower of False Horn Plantain (Musa AAB). Plant Cell Tissue Organ Cult 61:237–244
- Jalil M, Khalid N, Othman RY (2008) Plant regeneration from embryogenic suspension cultures of Musa acuminata cv. Mas (AA). Plant Cell Tissue Organ Cult 75:209–214
- Johansen DA (1940) Plant microtechnique. McGraw Hill, New York
- Jonathon B, Chatfield S, Leyser O (2003) Auxin acts in xylem-associated or Medullary cells to mediate apical dominance. Plant Cell 15:495–507
- Khalil SM, Cheah KT, Perez EA, Gaskill DA, Hu JS (2002) Regeneration of banana (Musa spp. AAB cv. ‘Dwarf Brazilian’) via secondary somatic embryogenesis. Plant Cell Rep 20:1128–1134
- Lee KS, Zapita FJA, Brunner H, Afza R (1997) Histology os somatic embryo initiation and organogenesis from rhizome explants of Musa Spp. Plant Cell Tissue Organ Cult 51:1–8
- Morais-Lino LS, Serejo JAS, Amorim EP, de-Santana JRF, Pasqual M, Silva SD (2016) Somatic embryogenesis, cell suspension, and genetic stability of banana cultivars. Vitro Cell Dev Biol Plant 52(1):99–106
- Navarro C, Escobedo RM, Mayo A (1997) In vitro plant regeneration from embryogenic cultures of a diploid and a triploid, Cavendish banana. Plant Cell Tissue Organ Cult. 51:17–25
- Ngomuo M, Mneney E, Ndakidemi PA (2014) The in vitro propagation techniques for producing banana using shoot tip cultures. Am J Plant Sci 5:1614–1622
- Novak FJ, Afza R, van Duren M, Perra-Dallos M, Conger BV, Tang XL (1989) Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA and AAA) and cooking (ABB) banana (Musa spp.). Bio/Technology 7:154–159
- Quiroz-Figueroa F, Fuentes-Cerda C, Rojas-Herrera R, Loyola-Vargas V (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep 20:1141–1149
- Rayis SA, Abdallah AA (2015) Somatic embryogenesis for the genetic improvement of a triploid banana (Musa Spp. AAA Cv. Berangan) using three different media with different growth regulators. Int J Recent Res Life Sci 2:53–58
- Remakanthan A, Menon TG, Soniya EV (2014) Somatic embryogenesis in banana (Musa accuminata AAA cv. Grand Naine): effect of explant and culture conditions. Vitro Cell Dev Biol Plant 50:127–136
- Schoofs H, Panis B, Swennen R (1998) Competence of scalps for somatic embryogenesis in Musa. Proc Internat Sympos Banana Subtrop ISHS Acta Hortic 490:475–483
- Sholi NJY, Chaurasia A, Agrawal A, Sarin NB (2009) ABA enhances plant regeneration of somatic embryos derivedfrom cell suspension cultures of plantain cv. Spambia (Musa sp.). Plant Cell Tiss Organ Cult 99:133–140
- Sidha M, Suprasanna P, Bapat VA, Kulkarni UG, Shinde BN (2007) Developing somatic embryogenic culture system and plant regeneration in banana. BARC News Lett, Issue, p 285
- Singh HP, Uma S, Selvarajan R, Karihaloo JL (2011) Micropropagation for production of quality banana planting material in AsiaPacific. Asia-Pacific Consortium on Agricultural Biotechnology (APCoAB), New Delhi, p 92
- Smitha PD, Nair AS (2011) Effect of Picloram on Somatic Embryogenesis from Leaf Sheath Explants in Diploid Musa acuminata cv. Njalipoovan. Plant Tissue Cult Biotech 21:83–87
- Strosse H, Domergue R, Panis B, Escalant JV, Cote F (2003) Banana and plantain embryogenic cell suspensions (A.Vezina and C.Picq. editions). In: INIBAP Technical Guidelines 8. The International Network for the Improvement of Bananas and Plantain. Montpellier, France
- Strosse HI, Houwe VD, Panis B (2004) Cell and Tissue culture, and mutation induction in banana improvement: cellular, molecular biology, and induced mutations. Science Publishers, Enfield, pp 3–16 (Plymouth, UK )
- Strosse H, Schoofs H, Panis B, Andre E, Reyniers K, Swennen R (2006) Development of embryogenic cell suspensions from shoot meristematic tissue in bananas and plantains (Musa spp.). Plant Sci 170:104–112
- Thorpe TA, Stasolla C (2001) Somatic embryogenesis. In: Bhojwani SS, Soh WY (eds) Current trends in the embryology of angiosperms. Kluwer Academic Publishers, Norwell, pp 279–336. https://doi.org/10.1007/978-94-017-1203-3_12
- Tripathi JN, Oduor RO, Tripathi L (2015) A high throughput regeneration and transformation plantform for production of genetically modified banana. Front Plant Sci 6:1025
- Uma S, Lakshmi S, Saraswathi MS et al (2011) Plant Cell Tiss Organ Cult 105:105. https://doi.org/10.1007/s11240-010-9847-9
- West MA, Harada J (1993) Embryogenesis in higher plants: an overview. Plant Cell 5:1361–1369
- Youssef AM, James CA, Mosqueda AM, Ku-Cauich JR, Arango RG, Rosa María Escobedo RM (2010) Influence of genotype and age of explant source on the capacity for somatic embryogenesis of two Cavendish banana cultivars (Musa acuminata Colla, AAA). Afr J Biotech 9:2216–2223
Author Information
Department of Botany, Gargi College, University Of Delhi, New Delhi, India