Exploring the antifungal properties of tannin extracts from Olea europaea var. rougette leaves against Aspergillus spp.
Bennacer Amel, Sahir-Halouane Fatma, Aitslimane-Aitkaki Sabrina, Tihar-Benzina Farida, Oukali Zahia, Oliveira Ivo Vaz, Rahmouni Naima
Research Articles | Published: 08 March, 2024
First Page: 353
Last Page: 363
Views: 3375
Keywords: n Olea europaea var. rougetten , Antifungal, Tannins, n Aspergillusn , Germination inhibition, Chromatography
Abstract
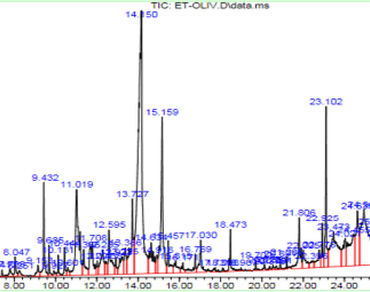
Due to significant health risks associated with chemical antifungal agents in food storage, safe and natural sources are now one of the most frequently demanded ingredient for food preservation. Byproducts from olive trees provide an affordable and sustainable source of bioactive chemicals. This study will examine the mycelial growth and germination-inhibitory properties of Olea europaea var. rougette leaves and characterize their phytochemical makeup.The phytochemical screening revealed the presence of polyphenolics compounds mainly tanins.The chemical composition was analyzed by gas chromatography mass spectrometry (GCMS). Total polyphenolic content (TPC), hydrolysable tannins content (HTC) and condensed tannin content (CTC) were assessed using Foilin ciocalteu, potassium iodide and vanilline methods, respectively. The mycelial growth and germination inhibition properties were examined on soft wheat based solid and liquid media. Tannic extract (ethyl acetate extract) revealed highest amounts in polyphenols, hydrolysable tanins and condensed. GC–MS profile of tannic extract showed the presence of 26 bioactive molecules known for their antifungal potential, mainly: Guaiacol (0.12%), 2-methoxy-4-vinylphenol (1.52%), Syringol (0.18%), p-tyrosol (4.70%), homovanillyl alcohol (1.32%), 4-propylresorcinol (1.07%), β-camphor (0.17%), β-turmerone (1.57%) and 4-phenyl-quinolin-2-ol (6.58%). The tannic extract (ethyl acetate fraction) showed significant mycelial growth and germination inhibition activities in contrast to hydro-acetonic and dichloromethanic extracts. Furthermore, myelial growth inhibition showed more effectiveness in comparison to germination inhibition technique.The results suggested that byproducts of Olea europaea var. rougette leaves are a substantial natural source of bioactive compounds, especially tannins with potent antifungal characteristics in vitro which make it interesting to investigate its effectiveness in vivo.
References
Abaza L, Taamalli A, Nsir H, Zarrouk M (2015) Olive tree (Olea europeae L.) leaves: importance and advances in the analysis of phenolic compounds. Antioxidants (basel) 4(4):682–698. https://doi.org/10.3390/antiox4040682
Akhtar MF, Ashraf KM, Saleem A, Sharif A, Zubair HM, Anwar F (2022) Antidiabetic potential and antioxidant activity of Olea europaea subsp. Cuspidata (Indian Olive) seed extracts. Evid Based Complement Altern Med 2022:5164985. https://doi.org/10.1155/2022/5164985
Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K (2001) Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother 48(4):487–491. https://doi.org/10.1093/jac/48.4.487
Arulappan MT, Britto JS, Ruckmani K, Kumar MR (2015) Antimicrobial and antifungal activities of Zehneria scabra (LF) sond against human pathogens. Int J Dev Res 5:3852–3859
Baazeem A, Rodriguez A, Medina A, Magan N (2021) Impacts of climate change interacting abiotic factors on growth, aflD and aflR gene expression and aflatoxin B1 production by Aspergillus flavus strains in vitro and on pistachio nuts. Toxins (basel) 13(6):385. https://doi.org/10.3390/toxins13060385.PMID:34071166;PMCID:PMC8228473
Bennacer A, Sahir-Halouane F, Aitslimane-Aitkaki S, Oukali Z, Oliveira IV, Rahmouni N, Aissaoui M (2022) Structural characterization of phytochemical content, antibacterial, and antifungal activities of Juglans regia L. leaves cultivated in Algeria. Biocatal Agric Biotechnol 40:102304. https://doi.org/10.1016/j.bcab.2022.102304
Borjan D, Leitgeb M, Knez Ž, Hrnčič MK (2020) Microbiological and antioxidant activity of phenolic compounds in olive leaf extract. Molecules 25(24):5946. https://doi.org/10.3390/molecules25245946
Cabañes FJ, Bragulat MR (2018) Black aspergilla and ochratoxin A-producing species in foods. Curr Opin Food Sci 23:1–10
Clodoveo ML, Crupi P, Annunziato A, Corbo F (2021) Innovative extraction technologies for development of functional ingredients based on polyphenols from olive leaves. Foods 11(1):103. https://doi.org/10.3390/foods11010103
Cook RT, Braun U (2009) Conidial germination patterns in powdery mildews. Mycol Res 113(5):616–636. https://doi.org/10.1016/j.mycres.2009.01.010
Da Cruz CL, Delgado J, Patriarca A, Rodríguez A (2019) Differential response to synthetic and natural antifungals by Alternaria tenuissima in wheat simulating media: growth, mycotoxin production and expression of a gene related to cell wall integrity. Int J Food Microbiol 2(292):48–55. https://doi.org/10.1016/j.ijfoodmicro.2018.12.005
De la Ossa JG, Felice F, Azimi B, Salsano JE, Digiacomo M, Macchia M, Danti S, Di Stefano R (2019) Waste autochthonous tuscan olive leaves (Olea europaea var. Olivastra seggianese) as antioxidant source for biomedicine. Int J Mol Sci 20(23):5918. https://doi.org/10.3390/ijms20235918
Djenane D, Gómez D, Yangüela J, Roncalés P, Ariño A (2018) Olive leaves extract from Algerian oleaster (Olea europaea var. sylvestris) on microbiological safety and shelf-life stability of raw halal minced beef during display. Foods 8(1):10. https://doi.org/10.3390/foods8010010
Ebadzadsahrai G, Higgins Keppler EA, Soby SD, Bean HD (2020) Inhibition of fungal growth and induction of a novel volatilome in response to Chromobacterium vaccinii volatile organic compounds. Front Microbiol 20(11):1035. https://doi.org/10.3389/fmicb.2020.01035
El SN, Karakaya S (2009) Olive tree (Olea europaea) leaves potential beneficial effects on human health. Nutr Rev 67(11):632–638. https://doi.org/10.1111/j.1753-4887.2009.00248.x
Ellappan T, Ramar M, Manikrishnan R, Melepuram SG, Balaji P, Sekar VK, Chidambaram K (2022) Protective effect of Ceiba pentandra (L) Gaertn on CCl4-induced oxidative stress and liver damage in rats. Pharmacol Res Mod Chin Med 5:100196. https://doi.org/10.1016/j.prmcm.2022.100196
Esposito A, De Luca PF, Graziani V, D’Abrosca B, Fiorentino A, Scognamiglio M (2021) Phytochemical characterization of Olea europaea L. cultivars of Cilento National Park (South Italy) through NMR-based metabolomics. Molecules 26(13):3845. https://doi.org/10.3390/molecules26133845
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118. https://doi.org/10.1111/j.1365-294x.1993.tb00005
Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N (2012) Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—a review. Int J Mol Sci 13(3):3291–3340. https://doi.org/10.3390/ijms13033291
Harris RF, Sommers LE (1968) Plate-dilution frequency technique for assay of microbial ecology. Appl Microbiol 16(2):330–334. https://doi.org/10.1128/am.16.2.330-334.1968
Hashmi MA, Khan A, Hanif M, Farooq U, Perveen S (2015) Traditional uses, phytochemistry, and pharmacology of Olea europaea (Olive). Evid Based Complement Altern Med 2015:541591. https://doi.org/10.1155/2015/541591
Julca I, Marcet-Houben M, Cruz F, Gómez-Garrido J, Gaut BS, Díez CM, Gut IG, Alioto TS, Vargas P, Gabaldón T (2020) Genomic evidence for recurrent genetic admixture during the domestication of Mediterranean olive trees (Olea europaea L.). BMC Biol 18(1):148. https://doi.org/10.1186/s12915-020-00881-6
Kritikou E, Kalogiouri NP, Kolyvira L, Thomaidis NS (2020) Target and suspect HRMS metabolomics for the determination of functional ingredients in 13 varieties of olive leaves and drupes from Greece. Molecules 25(21):4889. https://doi.org/10.3390/molecules25214889
Kumar RM, Gayatri N, Sivasudha T, Ruckmani K (2017) Profiling of bioactive components present in Ziziphus mauritiana Lam for in-vitro antioxidant and in-vivo anti-inflammatory activities. Int Res J Pharm 8(9):19–24
Lee SH, Oh YT, Lee DY, Cho E, Hwang BS, Jeon J (2022) Large-scale screening of the plant extracts for antifungal activity against the plant pathogenic fungi. Plant Pathol J 38(6):685–691. https://doi.org/10.5423/PPJ.NT.07.2022.0098
Mahmoudi A, Hadrich F, Feki I, Ghorbel H, Bouallagui Z, Marrekchi R, Fourati H, Sayadi S (2018) Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct 9(6):3220–3234. https://doi.org/10.1039/c8fo00248g
Martínez L, Ros G, Nieto G (2018) Hydroxytyrosol: health benefits and use as functional ingredient in meat. Medicines (basel) 5(1):13. https://doi.org/10.3390/medicines5010013
Martínez-Navarro ME, Cebrián-Tarancón C, Oliva J, Salinas MR, Alonso GL (2021) Oleuropein degradation kinetics in olive leaf, and its aqueous extracts. Antioxidants (basel) 10(12):1963. https://doi.org/10.3390/antiox10121963
Martínez-Zamora L, Peñalver R, Ros G, Nieto G (2021) Olive tree derivatives and hydroxytyrosol: their potential effects on human health and its use as functional ingredient in meat. Foods 10(11):2611. https://doi.org/10.3390/foods10112611
Moccia F, Piscitelli A, Giovando S, Giardina P, Panzella L, d’Ischia M, Napolitano A (2020) Hydrolyzable vs. condensed wood tannins for bio-based antioxidant coatings: superior properties of quebracho tannins. Antioxidants (Basel) 9(9):804. https://doi.org/10.3390/antiox9090804
Musci M, Yao S (2017) Optimization and validation of Folin-Ciocalteu method for the determination of total polyphenol content of Pu-erh tea. Int J Food Sci Nutr 68(8):913–918. https://doi.org/10.1080/09637486.2017.1311844
Muzzalupo I, Badolati G, Chiappetta A, Picci N, Muzzalupo R (2020) In vitro antifungal activity of olive (Olea europaea) leaf extracts loaded in chitosan nanoparticles. Front Bioeng Biotechnol 3(8):151. https://doi.org/10.3389/fbioe.2020.00151
Navale V, Vamkudoth KR, Ajmera S, Dhuri V (2021) Aspergillus derived mycotoxins in food and the environment: prevalence, detection, and toxicity. Toxicol Rep 2(8):1008–1030. https://doi.org/10.1016/j.toxrep.2021.04.013
Olmo-García L, Kessler N, Neuweger H, Wendt K, Olmo-Peinado JM, Fernández-Gutiérrez A, Baessmann C, Carrasco-Pancorbo A (2018) Unravelling the distribution of secondary metabolites in Olea europaea L.: exhaustive characterization of eight olive-tree derived matrices by complementary platforms (LC-ESI/APCI-MS and GC-APCI-MS). Molecules 23(10):2419. https://doi.org/10.3390/molecules23102419
Palomo-Ríos E, Narváez I, Pliego-Alfaro F, Mercado JA (2021) Olive (Olea europaea L). genetic transformation: current status and future prospects. Genes (basel) 12(3):386. https://doi.org/10.3390/genes12030386
Peng K, Jin L, Niu YD, Huang Q, McAllister TA, Yang HE, Denise H, Xu Z, Acharya S, Wang S, Wang Y (2018) Condensed tannins affect bacterial and fungal microbiomes and mycotoxin production during ensiling and upon aerobic exposure. Appl Environ Microbiol 84(5):e02274-e2317. https://doi.org/10.1128/AEM.02274-17
Phillipson JD (2007) Phytochemistry and pharmacognosy. Phytochemistry 68(22–24):2960–2972. https://doi.org/10.1016/j.phytochem.2007.06.028
Pitt JI, Hocking AD (2009) Fungi and food spoilage. Elsevier, New York
Ramar MK, Henry LJK, Ramachandran S, Chidambaram K, Kandasamy R (2022) Ziziphus mauritiana Lam attenuates inflammation via downregulating NFκB pathway in LPS-stimulated RAW 264.7 macrophages & OVA-induced airway inflammation in mice models. J Ethnopharmacol 295:115445. https://doi.org/10.1016/j.jep.2022.115445
Rocha J, Borges N, Pinho O (2020) Table olives and health: a review. J Nutr Sci 2(9):e57. https://doi.org/10.1017/jns.2020.50
Rubab M, Chelliah R, Saravanakumar K, Barathikannan K, Wei S, Kim JR, Yoo D, Wang MH, Oh DH (2020) Bioactive potential of 2-methoxy-4-vinylphenol and benzofuran from Brassica oleracea L. var capitate f, rubra (Red Cabbage) on oxidative and microbiological stability of beef meat. Foods 9(5):568. https://doi.org/10.3390/foods9050568
Samtiya M, Matthews KR, Dhewa T, Puniya AK (2022) Antimicrobial resistance in the food chain: trends, mechanisms, pathways, and possible regulation strategies. Foods 11(19):2966. https://doi.org/10.3390/foods11192966
Top SM, Preston CM, Dukes JS, Tharayil N (2017) Climate Influences the content and chemical composition of foliar tannins in green and senesced tissues of Quercus rubra. Front Plant Sci 16(8):423. https://doi.org/10.3389/fpls.2017.00423
Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E (2021) Towards advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives. Microorganisms 9(10):2041. https://doi.org/10.3390/microorganisms9102041
Wei X, Cui S, Xie Y (2022) Synthesis and antibacterial properties of oligomeric dehydrogenation polymer from lignin precursors. Molecules 27(5):1466. https://doi.org/10.3390/molecules27051466.PMID:35268566;PMCID:PMC8911982
Willis RB, Allen PR (1998) Improved method for measuring hydrolyzable tannins using potassium iodate. Analyst 123:435–439. https://doi.org/10.1039/a706862j
World Health Organization WHO (2015) WHO estimates of the global burden of foodborne diseases: Foodborne disease burden epidemiology reference group 2007–2015. https://apps.who.int/iris/handle/10665/199350
Yuan H, Ma Q, Ye L, Piao G (2016) The traditional medicine and modern medicine from natural products. Molecules 21(5):559. https://doi.org/10.3390/molecules21050559
Zang D, Hamauru Y (2003) Phenolic compounds, ascorbic acid, carotenoids and antioxidant properties of grebe, red and yellow bell peppers. Food Agric Environ 1(2):22–27
Zhang J, Ye KP, Zhang X, Pan DD, Sun YY, Cao JX (2017) Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front Microbiol 4(7):2094. https://doi.org/10.3389/fmicb.2016.02094
Taniwaki MH, Pitt JI, Magan N (2018) Aspergillus species and mycotoxins: occurrence and importance in major food commodities. Curr Opin Food Sci 23. https://doi.org/10.1016/j.cofs.2018.05.008
Bruneton J (1999) Pharmacogosie, phytochimie, plantes médicinales. Technique & Documentation, Lavoisier, Paris, p 348
Khan MIR, Poor P, Janda T (2022) Salicylic acid: a versatile signaling molecule in plants. J Plant Growth Regul 41:1887–1890. https://doi.org/10.1007/s00344-022-10692-4
Chabasse D, Bouchara JP, DE Gentile L, Bruns S, Cimon B, Penn P (2002) Les moisissures d’intérêt médical. Cahier de formation n°25, Bioforma, pp 157–159
Hagerman AE (2002) Hydrolyzable Tannin structural chemistry. Tannin Handbook. Miami University, Miami, FL, USA
Author Information
Laboratory of Valorization and Conservation of Biological Ressources (VALCORE), Department of Biology, Faculty of Sciences, University of M’Hamed Bougara, Boumerdes, Algeria