Evaluation of phytochemical, antimicrobial and time-killing assay of Camellia species
Research Articles | Published: 01 September, 2020
First Page: 759
Last Page: 765
Views: 3906
Keywords: Phytochemicals, Antimicrobial, Agar diffusion, MIC, MBC, Time-killing
Abstract
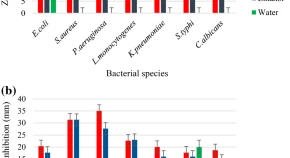
Tea (Camellia sinensis and C. assamica) possess antimicrobial property due to the presence of bioactive compounds. Qualitative analysis of phytochemicals of both tea species such as alkaloids, flavonoids, carbohydrates, saponins, tannins, steroids, terpenoids and cardiac glycosides was carried out. The antimicrobial activity of both plant species extracts were performed by well and disc agar diffusion methods. The most antimicrobial potential of plant extract was further selected to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against two most susceptible human pathogens. Phytochemicals and antimicrobial activity assays showed different results according to the type of the applied extract solvent. The methanol extract of C. sinensis shows more inhibitory effect than the other extracts of both tea species. The lowest concentration of C. sinensis was found at 0.048 mg/mL against Staphylococcus aureus. The MIC and MBC of S. aureus and Pseudomonas aeruginosa were ranged from 0.097 to 0.197 mg/mL and 0.19 to 0.39 mg/mL, respectively. Time-killing assay was performed against all tested pathogenic bacteria. After 6 h, P. aeruginosa showed no growth followed by Escherichia coli, Candida albicans, Klebsiella pneumoniae, S. aureus, Salmonella typhi, Listeria monocytogenes.
References
- Ayinde BA, Onwukaeme DN, Omogbai EKI (2007) Isolation and characterization of two phenolic compounds from the stem bark of Musanga cecropioides R. Brown (Moraceae). Acta Pol Pharm 64:183–185
- Baravalia Y, Kaneria M, Vaghasiya Y, Parekh J, Chanda S (2009) Antioxidant and antibacterial activity of Diospyros ebenum Roxb. leaf extracts. Turk J Biol 33(2):159–164
- Boyanova L, Gergova G, Nikolov R, Derejian S, Lazarova E, Katsarov N, Mitov I, Krastev Z (2005) Activity of Bulgarian propolis against Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J Med Microbiol 54:481–483
- Broadhurst CL, Polansky MM, Anderson RA (2000) Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem 48:894–952
- Cabrera C, Gimenez R, Lopez MC (2003) Determination of tea components with antioxidant activity. J Agric Food Chem 51:4427–4435
- Chacko SM, Thambi PT, Kuttan R, Nishigaki I (2010) Beneficial effects of green tea: a literature review. Chin Med 5(1):13
- Cho YS, Schiller NL, Oh KH (2008) Antibacterial effects of green tea polyphenols on clinical isolates of methicillin-resistant Staphylococcus aureus. Curr Microbiol 57(6):542–546
- Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH et al (2003) Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J 17(13):1913–1915
- Cushnie TT, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26(5):343–356
- Deba F, Xuan TD, Yasuda M, Tawata S (2008) Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control 19(4):346–352
- Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4(7):685–688
- Grange JM, Davey RW (1990) Antibacterial properties of Propolis (bee glue). J R Soc Med 83:159–160
- Hassan HM, Jiang ZH, Asmussen C, McDonald E, Qin W (2014) Antibacterial activity of northern Ontario medicinal plant extracts. Can J Plant Sci 94(2):417–424
- Hausteen BH (2005) The biochemistry and medical significance of the flavonoids. Pharmacol Ther J 96:67–202
- Igbinosa EO, Idemudia OG (2016) Anti-vibrio potentials of acetone and aqueous leaf extracts of Ocimum gratissimum (Linn). Trop J Pharm Res 15(4):743–750
- Inamdar P, Jelamvazir DS, Patel D, Meshram D (2014) Phytochemical screening and in vitro antifungal activity of Camellia sinensis. Int J Pharm Pharm Sci 6(5):148–150
- Infectious Diseases Society of America (2004) Bad bugs, no drugs; as antibiotic discovery stagnates, a public health crisis brews. Infectious Diseases Society of America, Alexandria, VA
- Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal-Okeng JW (2010) Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res 4(9):753
- Kelble A (2006) Spices and type 2 diabetes. Nutr Food Sci 35:81–87
- Li-Weber M (2009) New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituent Wogonin, Baicalein and Bacalin. Cancer Treat Rev 35:57–68
- McKay DL, Blumberg JB (2002) The role of tea in human health: an update. J Am Coll Nutr 21:1–13
- Mehrotra S, Srivastava AK, Nandi SP (2010) Comparative antimicrobial activities of Neem, Amla, Aloe, Assam Tea and Clove extracts against Vibrio cholerae, Staphylococcus aureus and Pseudomonas aeruginosa. J Med Plants Res 4(23):2473–2478
- Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2018) Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci 25(2):361–366
- Nazer A, Kobilinsky A, Tholozan JL, Dubois-Brissonnet F (2005) Combinations of food antimicrobials at low levels to inhibit the growth of Salmonellasv.Typhimurium: a synergistic effect? Food Microbiol 22(5):391–398
- Ngoci SN, Mwendia CM, Mwaniki CG (2011) Phytochemical and cytotoxicity testing of Indigofera lupatana Baker F. J Anim Plant Sci 11(1):1364–1373. https://www.biosciences.elewa.org/JAPS
- Okwu DE (1999) Flavouring properties of spices on cassava Fufu. Afr J Roots Tuber Crops 3(2):19–21
- Padmini E, Valarmathi A, Rani MU (2010) Comparative analysis of chemical composition and antibacterial activities of Mentha spicata and Camellia sinensis. Asian J Exp Biol Sci 1(4):772–781
- Reygaert WC (2018) Green tea catechins: their use in treating and preventing infectious diseases. BioMed Res Int 2018:9105261. https://doi.org/10.1155/2018/9105261
- Sesso H, Gaziano J, Buring J, Hennekens C (1999) Coffee and tea intake and the risk of myocardial infarction. Am J Epidemiol 149:162–167
Author Information
Department of Botany and Microbiology, Gurukula Kangri Vishwavidyalaya, Haridwar, India