Elucidation of natural compounds Gallic acid and Shikonin for the treatment of HNSC cancer by targeting immune suppressor and tumour progressor genes
Research Articles | Published: 21 March, 2022
First Page: 880
Last Page: 894
Views: 4350
Keywords: Head and neck squamous cell carcinoma (HNSC), Immunomodulation, Natural compounds, Gallic acid, Shikonin
Abstract
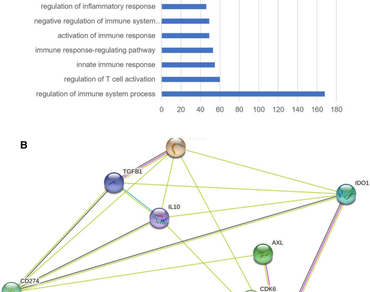
Head and Neck Squamous cell carcinoma is a leading cancer in males, especially in India. Progression of tumour growth in conjunction with immune suppression is the major cause that leads to HNSC cancer. Synthetic drugs targeting tumour cells often trigger tumour cells to acquire resistance against them. Immunotherapy also has its side effects and does not provide an adequate response in all patients, and its inherent variability in patient response often makes them prohibitive. Hence, a concomitant targeting of tumour cells and modulation of immune cell function may be particularly beneficial mechanism for cancer treatment. In the present study, we tried to identify natural compounds that could help in tumour suppression as well as functional immune modulation. The HNSC-associated genes that played role in both tumour growth and immune suppression were identified by enrichment analysis followed by gene expression analysis. 10 such genes were shortlisted, namely Foxp3, CD274, IDO1, IL-10, SOCS1, PRKDC, AXL, CDK6, TGFB1, FADD. CD274 and IDO1 which were found to have the highest degree of interaction based on their network of interactions. Gallic acid and Shikonin were found as the natural compounds that efficiently targeted CD274 and IDO1 respectively. Gallic acid is extracted from leaves of bearberry also found, in pomegranate root bark, gallnuts, witch hazel, both in free-state and as also a part of the tannin molecule, whereas Shikonin is found in the extracts of dried roots of the plant Lithospermum erythrorhizon. Studies have demonstrated that both Shikonin and Gallic acid exhibits anti-cancer properties. Expression data analysis of HNSC cancer exhibited 1745 differentially expressed genes. Gallic acid treatment resulted in the downregulation of 120 genes and upregulation of 35 genes while Shikonin treatment resulted in the downregulation of 660 genes and upregulation of 38 genes that are consequential in a positive impact of cancer regression. Thus, combination of these two compounds could be potentially beneficial in effective combinatorial therapy for HNSC.
References
Akinleye A, Rasool Z (2019) Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol 12(1):92. https://doi.org/10.1186/s13045-019-0779-5 (BioMed Central Ltd.)
Al Zahrani NA, El-Shishtawy RM, Asiri AM (2020) Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: a review. Eur J Med Chem 204:112609. https://doi.org/10.1016/J.EJMECH.2020.112609
Alfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AHH, Mohammed OY, Elhassan GO, Harguindey S, Reshkin SJ, Ibrahim ME, Rauch C (2015) Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int 15(1):71. https://doi.org/10.1186/s12935-015-0221-1 (BioMed Central Ltd.)
Alkhalaf MI, Alansari WS, Ibrahim EA, ELhalwagy MEA (2019) Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J King Saud Univ Sci 31(4):1358–1362. https://doi.org/10.1016/J.JKSUS.2018.10.010
Amin ARMR, Kucuk O, Khuri FR, Shin DM (2009) Perspectives for cancer prevention with natural compounds. J Clin Oncol off J Am Soc Clin Oncol 27(16):2712–2725. https://doi.org/10.1200/JCO.2008.20.6235
Aung TN, Qu Z, Kortschak RD, Adelson DL (2017) Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci. https://doi.org/10.3390/ijms18030656 (MDPI AG)
Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL (2009) IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 183(4):2475–2483. https://doi.org/10.4049/jimmunol.0900986
Bilir C, Sarisozen C (2017) Indoleamine 2,3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncol Sci 3(2):52–56. https://doi.org/10.1016/j.jons.2017.04.001
Bozic I, Nowak MA (2014) Timing and heterogeneity of mutations associated with drug resistance in metastatic cancers. Proc Natl Acad Sci USA 111(45):15964–15968. https://doi.org/10.1073/pnas.1412075111
Calon A, Espinet E, Palomo-Ponce S, Tauriello DVF, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XHF, Byrom D, Riera A, Rossell D, Mangues R, Massagué J, Sancho E, Batlle E (2012) Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22(5):571–584. https://doi.org/10.1016/j.ccr.2012.08.013
Chen L, Zeng Y, Zhou S-F (2018) Role of apoptosis in cancer resistance to chemotherapy. Curr Underst Apoptosis Program Cell Death. https://doi.org/10.5772/intechopen.80056 (InTech)
Chikuma S (2017) CTLA-4, an essential immune-checkpoint for T-cell activation. Curr Top Microbiol Immunol 410:99–126. https://doi.org/10.1007/82_2017_61 (Springer Verlag)
Chikuma S, Kanamori M, Mise-Omata S, Yoshimura A (2017) Suppressors of cytokine signaling: Potential immune checkpoint molecules for cancer immunotherapy. Cancer Sci 108(4):574–580. https://doi.org/10.1111/cas.13194 (Blackwell Publishing Ltd.)
Choi H, Cho SY, Pak HJ, Kim Y, Choi J, Lee YJ, Gong BH, Kang YS, Han T, Choi G, Cho Y, Lee S, Ryoo D, Park H (2017) NPCARE: database of natural products and fractional extracts for cancer regulation. J Cheminformatics 9(1):2. https://doi.org/10.1186/s13321-016-0188-5
Daglia M, Lorenzo A, Nabavi S, Talas Z, Nabavi S (2014) Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Curr Pharm Biotechnol 15(4):362–372. https://doi.org/10.2174/138920101504140825120737
Demir S, Ayazoglu Demir E, Turan I, Ozgen U (2021) Evaluation of cytotoxic effect of onosma armeniacum extract on various cancer cells. KSU J Agric Nat 24(2):252–259. https://doi.org/10.18016/ksutarimdoga.vi.729814
Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, Ivanova E, Paweletz CP, Bowden M, Zhou CW, Herter-Sprie GS, Sorrentino JA, Bisi JE, Lizotte PH, Merlino AA, Wong KK (2018) CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov 8(2):216–233. https://doi.org/10.1158/2159-8290.CD-17-0915
Desai P, Wallace R, Anderson ML, Howard BV, Ray R, Wu C, Safford M, Martin LW, Schlecht N, Liu S, Cirillo D, Jay A, Manson JAE, Simon MS (2018) An analysis of the effect of statins on the risk of Non-Hodgkin’s Lymphoma in the Women’s Health Initiative cohort. Cancer Med 7(5):2121–2130. https://doi.org/10.1002/cam4.1368
Dhandapani R, Sarkar AK (2007) Antibacterial activity and uv property of shikonin on silk substrate. J Text Appar Technol Manag 5(4):1–7
Ferris RL (2015) Immunology and immunotherapy of head and neck cancer. J Clin Oncol 33(29):3293–3304. https://doi.org/10.1200/JCO.2015.61.1509
Furler RL, Nixon DF, Brantner CA, Popratiloff A, Uittenbogaart CH (2018) TGF-β sustains tumor progression through biochemical and mechanical signal transduction. Cancers. https://doi.org/10.3390/cancers10060199 (MDPI AG)
Gaston TE, Mendrick DL, Paine MF, Roe AL, Yeung CK (2020) “Natural” is not synonymous with “Safe”: toxicity of natural products alone and in combination with pharmaceutical agents. Regul Toxicol Pharmacol RTP. https://doi.org/10.1016/J.YRTPH.2020.104642
George BP, Chandran R, Abrahamse H (2021) Role of phytochemicals in cancer chemoprevention: insights. Antioxidants 10(9):1455. https://doi.org/10.3390/ANTIOX10091455
Goel S, Decristo MJ, Watt AC, Brinjones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, Hoog J, Ellis MJ, Ma CX, Ramm S, Krop IE, Winer EP, Roberts TM, Kim HJ, McAllister SS, Zhao JJ (2017) CDK4/6 inhibition triggers anti-tumour immunity. Nature 548(7668):471–475. https://doi.org/10.1038/nature23465
Gomes CA, Girão da Cruz T, Andrade JL, Milhazes N, Borges F, Marques MPM (2003) Anticancer activity of phenolic acids of natural or synthetic origin: a structure−activity study. J Med Chem 46(25):5395–5401. https://doi.org/10.1021/jm030956v
Han Y, Liu D, Li L (2020) PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 10(3): 727–742. http://www.ncbi.nlm.nih.gov/pubmed/32266087
Harjunpää H, Guillerey C (2020) TIGIT as an emerging immune checkpoint. Clin Exp Immunol 200(2):108–119. https://doi.org/10.1111/cei.13407
Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z, Halmos G, Székvölgyi L (2018) The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front Immunol 9:31. https://doi.org/10.3389/fimmu.2018.00151 (Frontiers Media S.A)
Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Blumberg RS (2015) CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517(7534):386–390. https://doi.org/10.1038/nature13848
Kearney CJ, Ramsbottom KM, Voskoboinik I, Darcy PK, Oliaro J (2016) Loss of DNAM-1 ligand expression by acute myeloid leukemia cells renders them resistant to NK cell killing. OncoImmunology. https://doi.org/10.1080/2162402X.2016.1196308
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26(1):677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331
Knee DA, Hewes B, Brogdon JL (2016) Rationale for anti-GITR cancer immunotherapy. Eur J Cancer 67:1–10. https://doi.org/10.1016/j.ejca.2016.06.028 (Elsevier Ltd.)
Liu T, Guo F, Zhu X, He X, Xie L (2017) Thalidomide and its analogues: a review of the potential for immunomodulation of fibrosis diseases and opthalmopathy. Exp Ther Med 14(6):5251–5257. https://doi.org/10.3892/etm.2017.5209 (Spandidos Publications)
Llanes-Fernández L, Álvarez-Goyanes RI, del Arango-Prado MC, Alcocer-González JM, Mojarrieta JC, Pérez XE, López MO, Odio SF, Camacho-Rodríguez R, Guerra-Yi ME, Madrid-Marina V, Tamez-Guerra R, Rodríguez-Padilla C (2006) Relationship between IL-10 and tumor markers in breast cancer patients. Breast 15(4):482–489. https://doi.org/10.1016/j.breast.2005.09.012
Long L, Zhang X, Chen F, Pan Q, Phiphatwatchara P, Zeng Y, Chen H (2018) The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 9(5–6):176–189. https://doi.org/10.18632/genesandcancer.180
Ludwig KF, Du W, Sorrelle NB, Wnuk-Lipinska K, Topalovski M, Toombs JE, Cruz VH, Yabuuchi S, Rajeshkumar NV, Maitra A, Lorens JB, Brekken RA (2018) Small-molecule inhibition of Axl targets tumor immune suppression and enhances chemotherapy in pancreatic cancer. Can Res 78(1):246–255. https://doi.org/10.1158/0008-5472.CAN-17-1973
Mahdi SHA, Cheng H, Li J, Feng R (2015) The effect of TGF-beta-induced epithelial-mesenchymal transition on the expression of intracellular calcium-handling proteins in T47D and MCF-7 human breast cancer cells. Arch Biochem Biophys 583:18–26. https://doi.org/10.1016/j.abb.2015.07.008
Majolo F, de Oliveira Becker Delwing LK, Marmitt DJ, Bustamante-Filho IC, Goettert MI (2019) Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem Lett 31:196–207. https://doi.org/10.1016/j.phytol.2019.04.003
Marinelli O, Nabissi M, Morelli MB, Torquati L, Amantini C, Santoni G (2018) ICOS-L as a potential therapeutic target for cancer immunotherapy. Curr Protein Pept Sci 19(11):1107–1113. https://doi.org/10.2174/1389203719666180608093913
Masuda M, Wakasaki T, Toh S, Shimizu M, Adachi S (2011) Chemoprevention of head and neck cancer by green tea extract: EGCG-the role of EGFR signaling and “lipid raft.” J Oncol. https://doi.org/10.1155/2011/540148
Matsushita M, Kawaguchi M (2018) Immunomodulatory effects of drugs for effective cancer immunotherapy. J Oncol. https://doi.org/10.1155/2018/8653489
Mercer F, Unutmaz D (2009) The biology of FoxP3: AKey player in immune suppression during infections, autoimmune diseases and cancer. Adv Exp Med Biol 665:47–59. https://doi.org/10.1007/978-1-4419-1599-3_4
Miyata Y, ScienMatsuoces T, Araki K, Nakamura Y, Sagara Y, Ohba K, Sakai H (2018) Anticancer effects of green tea and the underlying molecular mechanisms in bladder cancer. Medicines 5(3):87. https://doi.org/10.3390/MEDICINES5030087
Nigorikawa K, Yoshikawa K, Sasaki T, Iida E, Tsukamoto M, Murakami H, Maehama T, Hazeki K, Hazeki O (2006) A naphthoquinone derivative, shikonin, has insulin-like actions by inhibiting both phosphatase and tensin homolog deleted on chromosome 10 and tyrosine phosphatases. Mol Pharmacol 70(3):1143–1149. https://doi.org/10.1124/MOL.106.025809
Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, Hsieh K, Cado D, Robey EA, Winoto A (2010) Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci USA 107(29):13034–13039. https://doi.org/10.1073/pnas.1005997107
Papoff G, Trivieri N, Crielesi R, Ruberti F, Marsilio S, Ruberti G (2010) FADD-calmodulin interaction: a novel player in cell cycle regulation. Biochim Et Biophys Acta Mol Cell Res 1803(8):898–911. https://doi.org/10.1016/j.bbamcr.2010.04.006
Pereira FV, Melo ACL, Low JS, de Castro ÍA, Braga TT, Almeida DC, de Lima AGUB, Hiyane MI, Correa-Costa M, Andrade-Oliveira V, Origassa CST, Pereira RM, Kaech SM, Rodrigues EG, Câmara NOS (2018) Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response. Oncotarget 9(40):25808–25825. https://doi.org/10.18632/oncotarget.25380
Platten M, Wick W, Van Den Eynde BJ (2012) Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res 72(21):5435–5440. https://doi.org/10.1158/0008-5472.CAN-12-0569
Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ (2014) Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 63(7):721–735. https://doi.org/10.1007/s00262-014-1549-4 (Springer Science and Business Media Deutschland GmbH)
Rankin EB, Giaccia AJ (2016) The receptor tyrosine kinase AXL in cancer progression. Cancers. https://doi.org/10.3390/cancers8110103 (MDPI AG)
Sakhnevych S (2019) Tim-3-galectin-9 immunosuppressive pathway in human acute myeloid leukaemia and solid tumour cells and biochemical functions of its crucial components
Salehan MR, Morse HR (2013) DNA damage repair and tolerance: a role in chemotherapeutic drug resistance. Br J Biomed Sci 70(1):31–40. https://doi.org/10.1080/09674845.2013.11669927 (Step Publishing Ltd.)
Shao L, Hou W, Scharping NE, Vendetti FP, Srivastava R, Roy CN, Menk AV, Wang Y, Chauvin JM, Karukonda P, Thorne SH, Hornung V, Zarour HM, Bakkenist CJ, Delgoffe GM, Sarkar SN (2019) IRF1 inhibits antitumor immunity through the upregulation of PD-L1 in the tumor cell. Cancer Immunol Res 7(8):1258–1266. https://doi.org/10.1158/2326-6066.CIR-18-0711
Sharma RA, Singh B, Singh D, Chandrawat P (2009) Ethnomedicinal, pharmacological properties and chemistry of some medicinal plants of Boraginaceae in India. J Med Plants Res 3(13): 1153–1175. http://www.academicjournals.org/JMPR
Sheikhpour E, Noorbakhsh P, Foroughi E, Farahnak S, Nasiri R, Neamatzadeh H (2018) A survey on the role of interleukin-10 in breast cancer: a narrative. Rep Biochem Mol Biol 7(1): 30–37. http://www.ncbi.nlm.nih.gov/pubmed/30324115
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, Von Mering C (2017) The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucl Acids Res 45(D1):D362–D368. https://doi.org/10.1093/nar/gkw937
Tadesse S, Yu M, Kumarasiri M, Le BT, Wang S (2015) Targeting CDK6 in cancer: State of the art and new insights. Cell Cycle 14(20):3220–3230. https://doi.org/10.1080/15384101.2015.1084445 (Taylor and Francis Inc.)
Tan KT, Yeh CN, Chang YC, Cheng JH, Fang WL, Yeh YC, Wang YC, Hsu DSS, Wu CE, Lai JI, Chang PMH, Chen MH, Lu ML, Chen SJ, Chao Y, Hsiao M, Chen MH (2020) PRKDC: new biomarker and drug target for checkpoint blockade immunotherapy. J Immunother Cancer. https://doi.org/10.1136/jitc-2019-000485
Tanegashima T, Togashi Y, Azuma K, Kawahara A, Ideguchi K, Sugiyama D, Kinoshita F, Akiba J, Kashiwagi E, Takeuchi A, Irie T, Tatsugami K, Hoshino T, Eto M, Nishikawa H (2019) Immune suppression by PD-L2 against spontaneous and treatment-related antitumor immunity. Clin Cancer Res 25(15):4808–4819. https://doi.org/10.1158/1078-0432.CCR-18-3991
Thaker AI, Rao MS, Bishnupuri KS, Kerr TA, Foster L, Marinshaw JM, Newberry RD, Stenson WF, Ciorba MA (2013) IDO1 metabolites activate β-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. https://doi.org/10.1053/j.gastro.2013.05.002
Tímár J, Csuka O, Remenár E, Répássy G, Kásler M (2005) Progression of head and neck squamous cell cancer. Cancer Metastasis Rev 24:107–127
Torres N, Regge MV, Secchiari F, Friedrich AD, Spallanzani RG, Raffo Iraolagoitia XL, Núñez SY, Sierra JM, Ziblat A, Santilli MC, Gilio N, Almada E, Lauche C, Pardo R, Domaica CI, Fuertes MB, Madauss KP, Hance KW, Gloger IS, Zwirner NW (2020) Restoration of antitumor immunity through anti-MICA antibodies elicited with a chimeric protein. J Immunother Cancer 8(1):233. https://doi.org/10.1136/jitc-2019-000233
Turovskaya O, Kim G, Cheroutre H, Kronenberg M, Madan R (2009) Interleukin 10 acts on regulatory t cells to maintain expression of the transcription factor foxp3 and suppressive function in mice with colitis. Nat Immunol 10(11):1178–1184. https://doi.org/10.1038/ni.1791
Valanciene E, Jonuskiene I, Syrpas M, Augustiniene E, Matulis P, Simonavicius A, Malys N (2020) Advances and prospects of phenolic acids production, biorefinery and analysis. Biomolecules 10(6):1–41. https://doi.org/10.3390/BIOM10060874
Vasan N, Baselga J, Hyman DM (2019) A view on drug resistance in cancer. Nature 575(7782):299–309. https://doi.org/10.1038/s41586-019-1730-1 (Nature Publishing Group)
Verma S, Singh A, Mishra A (2013) Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol 35(3):473–485. https://doi.org/10.1016/j.etap.2013.02.011
Wang J, Iannarelli R, Pucciarelli S, Laudadio E, Galeazzi R, Giangrossi M, Falconi M, Cui L, Navia AM, Buccioni M, Marucci G, Tomassoni D, Serini L, Sut S, Maggi F, Dall’Acqua S, Marchini C, Amici A (2020) Acetylshikonin isolated from Lithospermum erythrorhizon roots inhibits dihydrofolate reductase and hampers autochthonous mammary carcinogenesis in Δ16HER2 transgenic mice. Pharmacol Res 161:105123. https://doi.org/10.1016/J.PHRS.2020.105123
Wu Y, Chen W, Xu ZP, Gu W (2019) PD-L1 distribution and perspective for cancer immunotherapy—blockade, knockdown, or inhibition. Front Immunol. https://doi.org/10.3389/fimmu.2019.02022 (Frontiers Media S.A)
Xia S, Ji R, Xu Y, Ni X, Dong Y, Zhan W (2017) Twisted gastrulation BMP signaling modulator 1 regulates papillary thyroid cancer cell motility and proliferation. J Cancer 8(14):2816–2827. https://doi.org/10.7150/jca.18482
Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Zhang JJ, Li HB (2017) Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Mol Sci. https://doi.org/10.3390/IJMS18010096
Xue X, Liang XJ (2012) Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer 31(2):100–109. https://doi.org/10.5732/cjc.011.10326 (BioMed Central)
Yang L, Pang Y, Moses HL (2010) TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 31(6):220–227. https://doi.org/10.1016/j.it.2010.04.002 (NIH Public Access)
Yang S, Liu Y, Li MY, Ng CSH, Yang S, Wang S, Zou C, Dong Y, Du J, Long X, Liu LZ, Wan IYP, Mok T, Underwood MJ, Chen GG (2017) FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer 16(1):1–12. https://doi.org/10.1186/s12943-017-0700-1
Yazaki K (2017) Lithospermum erythrorhizon cell cultures: present and future aspects. Plant Biotechnol 34(3):131. https://doi.org/10.5511/PLANTBIOTECHNOLOGY.17.0823A
Yu W, Hua Y, Qiu H, Hao J, Zou K, Li Z, Hu S, Guo P, Chen M, Sui S, Xiong Y, Li F, Lu J, Guo W, Luo G, Deng W (2020) PD-L1 promotes tumor growth and progression by activating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis 11(7):1–16. https://doi.org/10.1038/s41419-020-2701-z
Zaal EA, Berkers CR (2018) The influence of metabolism on drug response in cancer. Front Oncol. https://doi.org/10.3389/fonc.2018.00500 (Frontiers Media S.A)
Zeb A (2021) Phenolic antioxidants in foods: chemistry, biochemistry and analysis. Phenol Antioxid Foods Chem Biochem Anal. https://doi.org/10.1007/978-3-030-74768-8
Zhang J, Li H, Yu J-P, Wang SE, Ren X-B (2012) Role of SOCS1 in tumor progression and therapeutic application. Int J Cancer 130(9):1971–1980. https://doi.org/10.1002/ijc.27318
Zhang Y, Yang WK, Wen GM, Tang H, Wu CA, Wu YX, Jing ZL, Tang MS, Liu GL, Li DZ, Li YH, Deng YJ (2019) High expression of PRKDC promotes breast cancer cell growth via p38 MAPK signaling and is associated with poor survival. Mol Genet Genomic Med. https://doi.org/10.1002/mgg3.908
Author Information
Department of Biotechnology, Delhi Technological University, Delhi, India