Effect of extraction methods on yield, total polyphenols and flavonoids contents and antioxidant activity of Anvillea garcinii subsp. radiata using spectrophotometric and electrochemical approaches
*Article not assigned to an issue yet
Oucheikh Lahcen, Youssefi Youssef, Ou-Ani Omar, Mou Abdelaziz Ait Sidi, Znini Mohamed, Oubair Ahmad, Mabrouk Elhoussine, Chebabe Driss
Research Articles | Published: 25 April, 2025
First Page: 0
Last Page: 0
Views: 959
Keywords: n Anvillea garcinii subsp, n Radiatan , Antioxidant activity, Extraction methods, Cyclic voltammetry
Abstract
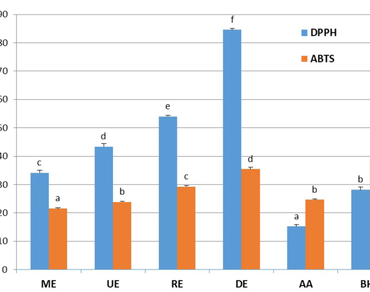
This study aims to determine the yield, and the total content of phenols and flavonoids as well as to evaluate the antioxidant activity of the decoction extract (DE) and three hydroethanolic extracts obtained by reflux heating (RE), maceration (ME) and ultrasound (UE) methods of Anvillea garcinii subsp. radiata. The antioxidant activity was assessed using spectrophotometric assays 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3) acid ethylbenzothiazoline-6-sulfonic acid (ABTS) and electrochemical cyclic voltammetry (CV) measurements. The results show that hydroethanolic extracts are rich in polyphenols and flavonoids and have better antioxidant activity than aqueous DE. The ME and UE showed interesting antioxidant activity, with respective IC50 values of 34.08 ± 0.92 and 43.39 ± 1.11 µg/ml for DPPH, and 21.50 ± 0.34 and 23.85 ± 0.14 µg/ml in ABTS assay. The RE recorded an IC50 of 53.97 ± 41 and 29.24 ± 0.48 µg/ml in the DPPH and ABTS assay, respectively. While the DE showed relatively low antioxidant activity, with an IC50 of 84.57 ± 0.58 µg/ml in the DPPH test. For total antioxidant capacity, CV test reveals that the ME, UE and RE extracts recorded interesting values of 40.68 ± 0.53, 32.79 ± 0.96 and 24.06 ± 0.59 mgAAE/g, respectively, while the DE value was around 11.63 ± 0.37 mgAAE/g.
References
Anderberg A (1982) The genus Anvillea (Compositae). Nord J Bot 2:297–305. https://doi.org/10.1111/j.1756-1051.1982.tb01193.x
Arribas AS, Martínez-Fernández M, Chicharro M (2012) The role of electroanalytical techniques in analysis of polyphenols in wine. TrAC Trends Anal Chem 34:78–96. https://doi.org/10.1016/j.trac.2011.10.015
Belhaoues S, Amri S, Bensouilah M (2020) Major phenolic compounds, antioxidant and antibacterial activities of Anthemis praecox link aerial parts. South Afr J Bot 131:200–205. https://doi.org/10.1016/j.sajb.2020.02.018
Benabid A (2000) Flore et écosystèmes du Maroc. Evaluation et préservation de la biodiversité
Benslama A, Harrar A, Gül F, Demirtaş I (2019) In vitro antioxidant, antibacterial activities and HPLC-TOF/MS analysis of Anvillea radiata (Asteraceae) extracts. Curr Nutr Food Sci 15:376–383. https://doi.org/10.2174/1573401314666171204161538
Blasco AJ, Rogerio MC, González MC, Escarpa A (2005) Electrochemical index as a screening method to determine total polyphenolics in foods: A proposal. Anal Chim Acta 539:237–244. https://doi.org/10.1016/j.aca.2005.02.056
Boukhris MA, Destandau É, El Hakmaoui A et al (2016) A dereplication strategy for the identification of new phenolic compounds from Anvillea radiata (Coss. & Durieu). Comptes Rendus Chim 19:1124–1132. https://doi.org/10.1016/j.crci.2016.05.019
Chiorcea-Paquim A-M, Enache TA, De Souza Gil E, Oliveira‐Brett AM (2020) Natural phenolic antioxidants electrochemistry: towards a new food science methodology. Compr Rev Food Sci Food Saf 19:1680–1726. https://doi.org/10.1111/1541-4337.12566
Choi Y, Lee J (2009) Antioxidant and antiproliferative properties of a tocotrienol-rich fraction from grape seeds. Food Chem 114:1386–1390. https://doi.org/10.1016/j.foodchem.2008.11.018
Destandau E, Boukhris MA, Zubrzycki S et al (2015) Centrifugal partition chromatography elution gradient for isolation of sesquiterpene lactones and flavonoids from Anvillea radiata. J Chromatogr B 985:29–37. https://doi.org/10.1016/j.jchromb.2015.01.019
Dhanani T, Shah S, Gajbhiye NA, Kumar S (2017) Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem 10:S1193–S1199. https://doi.org/10.1016/j.arabjc.2013.02.015
Djouadi A, Lanez T, Boubekri C (2016) Evaluation of antioxidant activity and polyphenolic contents of two South Algerian eggplants cultivars. J Fundam Appl Sci 8:223–231. https://doi.org/10.4314/jfas.v8i2.3
Floegel A, Kim D-O, Chung S-J et al (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal 24:1043–1048. https://doi.org/10.1016/j.jfca.2011.01.008
Gil-Martín E, Forbes-Hernández T, Romero A et al (2022) Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem 378:131918. https://doi.org/10.1016/j.foodchem.2021.131918
Hamada D, Ladjel S (2015) Chemical composition, in-vitro anti-microbial and antioxidant activities of the methanolic extract of Anvillea radiata Asteraceae. Res J Pharma Biol Chem Sci 6:1367–1373
Hebi M, Eddouks M (2018) Glucose Lowering activity of Anvillea radiata Coss & Durieu in diabetic rats. Cardiovasc haematol Disord-Drug targets former curr drug targets-. Cardiovasc Hematol Disord 18:71–80. https://doi.org/10.2174/1871529X18666180223100427
Higgins LM, Llanos E (2015) A healthy indulgence? Wine consumers and the health benefits of wine. Wine Econ Policy 4:3–11. https://doi.org/10.1016/j.wep.2015.01.001
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856. https://doi.org/10.1021/jf030723c
Ibtissam L, Boutlilis DA (2023) Interaction study of Greenly synthesized silver nanoparticles with bovine serum albumin (BSA) using spectrophotometric and voltammetric assays. Curr Trends Biotechnol Pharm 17:1013–1019. https://doi.org/10.5530/ctbp.2023.3.39
Jara-Palacios MJ, Escudero-Gilete ML, Hernández-Hierro JM et al (2017) Cyclic voltammetry to evaluate the antioxidant potential in winemaking by-products. Talanta 165:211–215. https://doi.org/10.1016/j.talanta.2016.12.058
Jha AK, Sit N (2022) Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci Technol 119:579–591. https://doi.org/10.1016/j.tifs.2021.11.019
Kandouli C, Cassien M, Mercier A et al (2017) Antidiabetic, antioxidant and anti inflammatory properties of water and n-butanol soluble extracts from Saharian Anvillea radiata in high-fat-diet fed mice. J Ethnopharmacol 207:251–267. https://doi.org/10.1016/j.jep.2017.06.042
Kilmartin PA, Zou H, Waterhouse AL (2001) A Cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J Agric Food Chem 49:1957–1965. https://doi.org/10.1021/jf001044u
Kumar G, Karthik L, Rao KVB (2013) Phytochemical composition and in vitro antioxidant activity of aqueous Extract of Aerva lanata (L.) Juss. Ex Schult. Stem (Amaranthaceae). Asian Pac J Trop Med 6:180–187. https://doi.org/10.1016/S1995-7645(13)60020-6
Lakhdar M, Meriem KH, Larbi B et al (2013) Phytochemical analysis and antifungal activity of Anvillea radiata. World Appl Sci J 26:165–171. https://doi.org/10.5829/idosi.wasj.2013.26.02.12112
Li H-B, Cheng K-W, Wong C-C et al (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem 102:771–776. https://doi.org/10.1016/j.foodchem.2006.06.022
Mohamed B, Khalid S, Tariq BED et al (2015) Bioactivity of Anvillea radiata Coss & dur. Collected from the Southeast of Morocco. Eur Sci J 11
Moharram HA, Youssef MM (2014) Methods for determining the antioxidant activity: a review. Alex J Food Sci Technol 11:31–42
Moumou M, El Bouakher A, Allouchi H et al (2014) Synthesis and biological evaluation of 9α-and 9β-hydroxyamino-parthenolides as novel anticancer agents. Bioorg Med Chem Lett 24:4014–4018. https://doi.org/10.1016/j.bmcl.2014.06.019
Munteanu IG, Apetrei C (2021) Analytical methods used in determining antioxidant activity: A review. Int J Mol Sci 22:3380. https://doi.org/10.3390/ijms22073380
Nirmala C, Bisht MS, Bajwa HK, Santosh O (2018) Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci Technol 77:91–99. https://doi.org/10.1016/j.tifs.2018.05.003
Olszowy M, Dawidowicz AL (2018) Is it possible to use the DPPH and ABTS methods for reliable Estimation of antioxidant power of colored compounds? Chem Pap 72:393–400. https://doi.org/10.1007/s11696-017-0288-3
Oreopoulou A, Tsimogiannis D, Oreopoulou V (2019) Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction parameters. Polyphenols Plants 243–259. https://doi.org/10.1016/B978-0-12-813768-0.00025-6
Oucheikh L, Ou-Ani O, Moujane S et al (2023) Chemical composition, in vitro antifungal activity, DFT, molecular docking and molecular dynamics simulation studies of the essential oil from Anvillea gracinii subsp. radiata (Coss. & Durieu) anderb. J Essent Oil Res 35:35–50. https://doi.org/10.1080/10412905.2022.2109767
Pisoschi AM, Pop A, Cimpeanu C, Predoi G (2016) Antioxidant capacity determination in plants and plant-derived products: A review. Oxid Med Cell Longev 2016. https://doi.org/10.1155/2016/9130976
Platzer M, Kiese S, Herfellner T et al (2021) Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 26:1244. https://doi.org/10.3390/molecules26051244
Ragubeer N, Beukes DR, Limson JL (2010) Critical assessment of voltammetry for rapid screening of antioxidants in marine algae. Food Chem 121:227–232. https://doi.org/10.1016/j.foodchem.2009.11.076
Saoud DH, Jelassi A, Hlila MB et al (2019) Biological activities of extracts and metabolites isolated from Anvillea radiata Coss. & Dur.(Asteraceae). South Afr J Bot 121:386–393. https://doi.org/10.1016/j.sajb.2018.10.033
Shen L, Pang S, Zhong M et al (2023) A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: principles, advantages, equipment, and combined technologies. Ultrason Sonochem 106646. https://doi.org/10.1016/j.ultsonch.2023.106646
Stevanović M, Stevanović S, Mihailović M et al (2020) Antioxidant capacity of dark red corn–Biochemical properties coupled with electrochemical evaluation. Rev Chim 71:31–41. https://doi.org/10.37358/RC.20.6.8167
Suzery M, Nudin B, Bima DN, Cahyono B (2020) Effects of temperature and heating time on degradation and antioxidant activity of anthocyanin from roselle petals (Hibiscus Sabdariffa L). Int J Sci Technol Manag 1:288–238. https://doi.org/10.46729/ijstm.v1i4.78
Turkoglu A, Duru ME, Mercan N et al (2007) Antioxidant and antimicrobial activities of laetiporus sulphureus (Bull.) murrill. Food Chem 101:267–273. https://doi.org/10.1016/j.foodchem.2006.01.025
Zielińska D, Turemko M (2020) Electroactive phenolic contributors and antioxidant capacity of flesh and Peel of 11 Apple cultivars measured by Cyclic voltammetry and HPLC–DAD–MS/MS. Antioxidants 9:1054. https://doi.org/10.3390/antiox9111054
Author Information
Laboratory of Materials Engineering for the Environment & Natural Resources, Faculty of Science and Technology, University Moulay Ismail of Meknes, Errachidia, Morocco