Distribution of endophytic fungi associated with Meriandra bengalensis Benth. and assessment of their bioactive potential in vitro
Research Articles | Published: 15 April, 2022
First Page: 995
Last Page: 1006
Views: 3352
Keywords: Fungal endophytes, Seasonal variation, Biocontrol, GC–MS analysis, Antibacterial activity
Abstract
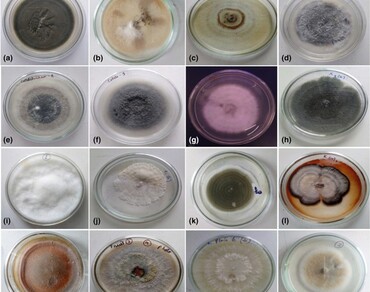
Fungal endophytes are known to be diverse and establish a mutualistic relationship with the host plant and protect them from the soil borne pathogens. In this study, we isolated a total of 283 fungal strains belonging to 8 different genera and 16 morphotypes from 450 tissue segments (Leaf, stems and flowers) of bengle sage (Meriandra bengalensis Benth.) plants during different (winter, summer and rainy) seasons, grown under natural conditions of Manipur and identified them on the basis of cultural morphology. The percentage of isolation rate (IR %) and colonization rates (CR %) of endophtic fungi communities were high in leaf segments (51% and 66%) and winter season (47% and 75%), respectively. The highest relative abundance (RA %) and isolation frequency (IF %) were recorded with Colletotrichum sp.2 and diversity indices of fungal endophytes were also calculated. The bioactive potential of Phoma sp. MBS13 was evaluated in vitro by dual culture tests against Aspergillus flavus, Aspergillus niger, Alternaria solani and Fusarium oxysporum isolates, which showed strong (Class 1and Class 2) antagonistic activity. In addition, the spectral (FTIR and GC–MS) analysis of ethyl acetate extracted metabolites of Phoma sp. MBS13 revealed the presence of various volatile compounds, and showed a good inhibitory activity against some human pathogenic bacteria i.e. Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Salmonella typhi. Maximum inhibitory zone (15.3 ± 0.15 mm) was recorded with E. coli. Thus, the present findings reveal the importance of endophytic fungi for their role in antimicrobial activity and biocontrol potential against pathogenic microorganisms that could be exploited by the biotechnological and agricultural industries.
References
Banerjee D, Manna S, Mahapatra S, Pati BR (2009) Fungal endophytes in three medicinal plants of Lamiaceae. Acta Microbiol Immunol Hung 56:243–250
Bell D, Wells KH, Markham CR (1982) In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 72:379–382
Bruno M, Mellerio G, Piozzi F, Vita-Finzi P (1985) GC-MS analysis of the essential oil of Meriandra benghalensis. In: Svendsen AB, Scheffer JJC (eds) Essential oils and aromatic plants. Martinus Nijhoff/Dr. W. Junk Publishers, Dordrecht, pp 151–154
Chowdhary K, Kaushik N (2015) Fungal endophyte diversity and bioactivity in the Indian Medicinal plant Ocimum sanctum L. PLoS One 10:e0141444. https://doi.org/10.1371/journal.pone.0141444
Debbab A, Aly AH, Edrada-Ebel RA, Müller WEG, Mosaddak M, Hakiki A, Ebel R, Proksch P (2009) Bioactive secondary metabolites from the endophytic fungus Chaetomium sp. isolated from Salvia officinalis growing in Morocco. Biotechnol Agron Soc Environ 13:229–234
Deka D, Jha DK (2020) Bioactivity assessment of endophytic fungi associated with Citrus macroptera Montr.: an endangered ethnomedicinal plant used in folk medicines in North-East India. Indian Phytopathol 73:21–33. https://doi.org/10.1007/s42360-019-00179-w
Gangadevi V, Muthumary J (2007) Endophytic fungal diversity from young, mature and senescent leaves of Ocimum basilicum L. with special reference to taxol production. Indian J Sci Technol 1:1–15
Gautam AK (2014) Diversity of fungal endophytes in some medicinal plants of Himachal Pradesh, India. Arch Phytopathol Plant Protect 47:537–544
Gomes da Silva GBP, Silvino KF, Bezerra JDP, de Farias TGS, de Araújo JM, Stamford TLM (2017) Antimicrobial activity of Phoma sp. URM 7221: an endophyte from Schinus terebinthifolius Raddi (Anacardiaceae). Afr J Microbiol Res 11:1–7
Göre ME, Bucak C (2007) Geographical and seasonal influence on the distribution of fungal endophytes in Laurus nobilis. For Pathol 37:281–288
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:4–9
Huang WY, Cai YZ, Hyde KD, Croke H, Sun M (2008) Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers 33:61–75
Jayanthi G, Karthikeyan K, Muthumary J (2014) Pervasiveness of endophytic fungal diversity in Anisomeles malabarica from Aliyar, Western Ghats, South India. Mycosphere 5:830–840
Kanjana M, Kanimozhi G, Udayakumar R, Panneerselvam A (2019) GC-MS analysis of bioactive compounds of endophytic fungi Chaetomium globosum, Cladosporium tenuissimum and Penicillium janthinellum. J Biomed Pharm Sci 2:1000123
Katoch M, Phull S, Vaid S, Singh S (2017) Diversity, phylogeny, anticancer and antimicrobial potential of fungal endophytes associated with Monarda citriodora L. BMC Microbiol 17:1–13
Kim JW, Choi HG, Song JH, Kang KS, Shim SH (2019) Bioactive secondary metabolites from an endophytic fungus Phoma sp. PF2 derived from Artemisia princeps Pamp. J Antibiot 72:174–177. https://doi.org/10.1038/s41429-018-0131-2
Krishnamurthy YL, Shankar NB, Shashikala J (2008) Fungal communities in herbaceous medicinal plants, Malnad region, Southern India. Microbes Environ 23:24–28
Masaumi S, Mirzaei S, Zafari D, Kalvandi R (2015) Isolation, identification and biodiversity of endophytic fungi of Thymus. Prog Biol Sci 5:43–50
Mishra A, Gond SK, Kumar A, Sharma VK, Verma SK, Kharwar RN, Sieber TN (2012) Season and tissue type affect fungal endophyte communities of the Indian medicinal plant Tinospora cordifolia more strongly than geographic location. Microb Ecol 64:388–398
Nirjanta Devi N, Singh MS (2013) GC-MS analysis of metabolites from endophytic fungus Colletotrichum gloeosporioides isolated from Phlogacanthus thyrsiflorus Nees. Int J Pharm Sci Rev Res 23:392–395
Paul NC, Deng J, Sang H, Choi YP, Yu SH (2012) Distribution and antifungal activity of endophytic fungi in different growth stages of chilli pepper (Capsicum annuum L.) in Korea. Plant Pathol J 28:10–19
Rajagopal R, Suryanarayann TS (2000) Isolation of endophytic fungi from leaves of neem (Azardirachta indica). Curr Sci 78:1375–1378
Rajagopal K, Kathireshan AK, Mahendran TS, Ananthi M, Seethalakshmi T (2008) Anti fungal activity endophytic and phellophytic fungi isolated from Neem (Azardirachta indica) against few pathogenic fungi and phylloplane fungi. Indian J Appl Microbiol 8:57–59
Rana VS, Blazquez MA (2009) Constituents of the essential oil of Meriandra bengalensis Benth. leaves from India. J Essent Oil Res 21:22–23
Rosa LH, Vieira MLA, Santiago IF, Rosa CA (2010) Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiol Ecol 73:178–189
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Simpson GG (1964) Species density of North American recent mammals. Syst Zool 13:57–73
Sinha SC (1996) Medicinal plants of Manipur. Mass & Sinha, Imphal, India, p 114
Strobel GA, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbial Mol Biol Rev 67:491–502
Suryanarayanan TS, Murali TS, Venkatesan G (2002) Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Botany 80:818–826
Wang LW, Xu BG, Wang JY, Su ZZ, Lin FC, Zhang CL, Kubicek CP (2012) Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl Microbiol Biotechnol 93:1231–1239. https://doi.org/10.1007/s00253-011-3472-3
Wearn JA, Sutton BC, Morley NJ, Gange AC (2012) Species and organ specificity of fungal endophytes in herbaceous grassland plants. J Ecol 100:1085–1092. https://doi.org/10.1111/j.1365-2745,2012.01997.x
Yu J, Wu Y, He Z, Li M, Zhu K, Gao B (2018) Diversity and antifungal of endophytic fungi associated with Camellia oleifera. Microbiology. https://doi.org/10.1080/12298093.2018.1454008
Author Information
Department of Life Sciences, Manipur University, Imphal, India