Decontamination of nodal segments from four native fruit species from Brazil related to sonication, asepsis time, and the use of antibiotics
*Article not assigned to an issue yet
Gomes Letícia Frabetti Cardoso de Mello Tucunduva, Stipp Liliane Cristina Liborio, Mourão Filho Francisco de Assis Alves
Research Articles | Published: 27 March, 2025
First Page: 0
Last Page: 0
Views: 805
Keywords: n Eugenia involucratan , n Eugenia brasiliensisn , n Eugenia pyriformisn , n Campomanesia phaean , In vitro propagation
Abstract
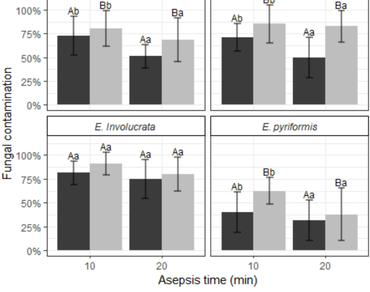
Myrtaceae is one of Brazil’s most significant plant families, including fruit trees and shrubs with a crucial role in the maintenance of the ecosystem. Although many Myrtaceous have agronomic potential, the production of nursery trees faces challenges due to seed recalcitrancy and low success on traditional vegetative multiplication methods. Micropropagation is a promising alternative, however, high contamination rates pose a significant barrier. This study evaluated the effectiveness of sonication, asepsis time, and the use of antibiotics in the decontamination of nodal segments from four native Myrtaceae fruit tree species: Campomanesia phaea, Eugenia brasiliensis, E. involucrata, and E. pyriformis. Sonication combined with a 20-minute asepsis treatment in NaOCl efficiently enhanced the control of fungal contamination, with no negative impact on oxidation and sprout emergence. Bacterial contamination was best controlled through the supplementation with antibiotics to the culture medium, with 500 mg L⁻¹ cefotaxime and a combination of 250 mg L⁻¹ cefotaxime and 200 mg L⁻¹ Timentin® being the most effective treatments.
References
Abreu-Tarazi MF, Navarrete AA, Andreote FD et al (2010) Endophytic bacteria in long-term in vitro cultivated axenic pineapple microplants revealed by PCR–DGGE. World J Microbiol Biotechnol 26:555–560. https://doi.org/10.1007/s11274-009-0191-3
Amorim IP, Silva JPN, Barbedo CJ (2020) As Sementes de Eugenia spp. (Myrtaceae) e Seus Novos conceitos sobre propagação. Hoehnea 47:e292020. https://doi.org/10.1590/2236-8906-29/2020
Cardoso JC, Teixeira da Silva JA (2013) Gerbera micropropagation. Biotechnol Adv 31:1344–1357. https://doi.org/10.1016/j.biotechadv.2013.05.008
de Carvalho ACPP, Rodrigues AA, de Santos J (2016) O Panorama da produção de mudas micropropagadas no Brasil (2008–2015)
Carvalho JMFC (1999) Técnicas de micropropagação, 1st edn. Embrapa Algodão, Campina Grande
da Silva APG, Sganzerla WG, Jacomino AP et al (2022) Chemical composition, bioactive compounds, and perspectives for the industrial formulation of health products from Uvaia (Eugenia pyriformis Cambess – Myrtaceae): A comprehensive review. J Food Compos Anal 109:104500. https://doi.org/10.1016/j.jfca.2022.104500
De Almeida CV, Andreote FD, Yara R et al (2009) Bacteriosomes in axenic plants: endophytes as stable endosymbionts. World J Microbiol Biotechnol 25:1757–1764. https://doi.org/10.1007/s11274-009-0073-8
Demétrio CA, de Oliveira Jacob JF, Ambrosano GB et al (2021) In vitro propagation of cambuci (Campomanesia phaea): an endangered exotic fruit and ornamental plant from Brazilian Atlantic forest. Plant Cell Tiss Organ Cult 145:203–208. https://doi.org/10.1007/s11240-020-02002-1
Duhan P, Bansal P, Rani S (2020) Isolation, identification and characterization of endophytic bacteria from medicinal plant Tinospora cordifolia. South Afr J Bot 134:43–49. https://doi.org/10.1016/j.sajb.2020.01.047
El-Banna AN, El-Mahrouk ME, Dewir YH et al (2021) Endophytic bacteria in banana in vitro cultures: molecular identification, antibiotic susceptibility, and plant survival. Horticulturae 7. https://doi.org/10.3390/horticulturae7120526
Falkiner FR (1997) Antibiotics in plant tissue culture and Micropropagation — What are we aiming at? In: Cassells AC (ed) Pathogen and microbial contamination management in micropropagation. Springer Netherlands, Dordrecht, pp 155–160
Fang J-Y, Hsu Y-R (2012) Molecular identification and antibiotic control of endophytic bacterial contaminants from micropropagated Aglaonema cultures. Plant Cell Tiss Organ Cult 110:53–62. https://doi.org/10.1007/s11240-012-0129-6
Gaba V, Kathiravan K, Amutha S et al (2008) The uses of ultrasound in plant tissue culture. Plant Tissue Cult Eng 417–426. https://doi.org/10.1007/978-1-4020-3694-1_22
Gallon FI, da Silva PRD, Silva DC et al (2018) da, Explants sterilization through metal nanoparticles for in vitro mass propagation of Eugenia involucrata. Plant Cell Culture & Micropropagation - ISSN 1808–9909 14:45–55
George EF, Hall MA, Klerk G-JD (2008) Plant tissue culture Procedure - Background. In: George EF, Hall MA, Klerk G-JD (eds) Plant propagation by tissue culture: volume 1. The background. Springer Netherlands, Dordrecht, pp 1–28
Girardelo JR, Munari EL, Dallorsoleta JCS et al (2020) Bioactive compounds, antioxidant capacity and antitumoral activity of ethanolic extracts from fruits and seeds of Eugenia involucrata DC. Food Res Int 137:109615. https://doi.org/10.1016/j.foodres.2020.109615
Goelzer A, Déo TG, Lopes GB, Damiani CR (2019) Reguladores de Crescimento Na multiplicação in vitro de Campomanesia adamantium (Cambess.) O. Berg (Myrtaceae) / Growth regulators in vitro multiplication of Campomanesiaadamantium (Cambess.)O. Berg (Myrtaceae). Brazilian Appl Sci Rev 3:1280–1291. https://doi.org/10.34115/basr.v3i2.1342
Golle DP, Reiniger LRS, Bellé RA, Curti AR (2013) Desinfestação superficial de explantes Isolados de Ramos semilenhosos e herbáceos de Eugenia involucrata DC. (Myrtaceae). CERNE 19:77–82. https://doi.org/10.1590/S0104-77602013000100010
Golle DP, Reiniger LRS, Stefanel CM et al (2020) Fitorreguladores e luminosidade Na indução à Calogênese Em explantes foliares de Eugenia involucrata DC. Ciênc Florest 30:898–906. https://doi.org/10.5902/1980509826987
Gressler E, Pizo MA, Morellato LPC (2006) Polinização e dispersão de Sementes Em myrtaceae do Brasil. Braz J Bot 29:509–530. https://doi.org/10.1590/S0100-84042006000400002
Grzebelus E, Skop L (2014) Effect of β-lactam antibiotics on plant regeneration in Carrot protoplast cultures. Vitro CellDevBiol-Plant 50:568–575. https://doi.org/10.1007/s11627-014-9626-0
Hesami M, Daneshvar MH, Lotfi-Jalalabadi A (2017) Effect of sodium hypochlorite on control of in vitro contamination and seed germination of ficus religiosa. Iran J Plant Physiol 7:2157–2162. https://doi.org/10.30495/ijpp.2017.537980
Jacomino AP, da Silva APG, de Freitas TP, de Paula Morais VS (2018) Uvaia—Eugenia pyriformis Cambess. In: Rodrigues S, de Oliveira Silva E, de Brito ES (eds) Exotic Fruits. Academic Press, pp 435–438
Leite MCDBS, Pereira APDA, Souza AJD et al (2018) Bioprospection and genetic diversity of endophytic bacteria associated with cassava plant. Rev Caatinga 31:315–325. https://doi.org/10.1590/1983-21252018v31n207rc
Leone GF, Andrade PAM, de Almeida CV et al (2019) Use of antibiotics to control endophytic bacterial growth migration onto culture medium in Eucalyptus cloeziana F.Muell.: a micropropagation approach. Vitro CellDevBiol-Plant 55:421–432. https://doi.org/10.1007/s11627-019-09986-2
Liu L-H, Yuan T, Zhang J-Y et al (2022) Diversity of endophytic bacteria in wild rice (Oryza meridionalis) and potential for promoting plant growth and degrading phthalates. Sci Total Environ 806:150310. https://doi.org/10.1016/j.scitotenv.2021.150310
Machado JS, Degenhardt J, Maia FR, Quoirin M (2020) Micropropagation of Campomanesia Xanthocarpa O. Berg (Myrtaceae), a medicinal tree from the Brazilian Atlantic forest. Trees 34:791–799. https://doi.org/10.1007/s00468-020-01958-z
Mongkolsook Y, Sumkaew R, Lichittammanit P et al (2007) In vitro micropropagation of agarwood (Aquilaria crassna). Proceedings of the 45th Kasetsart University Annual Conference, Bangkok, Thailand, 30 January-2 February 2007 Subject: Plants 532–538
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Myers N, Mittermeier RA, Mittermeier CG et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nascimento Ada, Paiva C, Nogueira R RC, et al (2008) BAP E AIB NO CULTIVO IN VITRO DE Eugenia pyriformis Cambess. Revista Acadêmica Ciência Anim 6:223–228. https://doi.org/10.7213/cienciaanimal.v6i2.10490
de Oliveira MLP, Costa MGC, da Silva CV, Otoni WC (2010) Growth regulators, culture media and antibiotics in the in vitro shoot regeneration from mature tissue of citrus cultivars. Pesq Agropec Bras 45:654–660. https://doi.org/10.1590/S0100-204X2010000700004
Oliveira Junior MA (2021) Cultivo in vitro de cerejeira-do-Rio-Grande (Eugenia involucrata DC.) e de Cambucizeiro (Campomanesia phaea (O. Berg) Landrum). Text, Universidade de São Paulo
Orlikowska T, Nowak K, Reed B (2017) Bacteria in the plant tissue culture environment. Plant Cell Tiss Organ Cult 128:487–508. https://doi.org/10.1007/s11240-016-1144-9
Palú EG, Corrêa L, de Suzuki S, Boliani AN AC (2011) Uso de antibióticos Para O controle de bactérias endógenas Visando à micropropagação Da Figueira. Rev Bras Frutic 33:587–592. https://doi.org/10.1590/S0100-29452011000200031
Pereira JES, de Fortes GR L (2003) Toxicidade de antibióticos no cultivo in vitro Da Batata Em meios semi-sólido e Líquido. Pesq Agropec Bras 38:1273–1279. https://doi.org/10.1590/S0100-204X2003001100004
Pereira MC, Steffens RS, Jablonski A et al (2012) Characterization and antioxidant potential of Brazilian fruits from the myrtaceae family. J Agric Food Chem 60:3061–3067. https://doi.org/10.1021/jf205263f
Qin YH, Teixeira da Silva JA, Bi JH et al (2011) Response of in vitro strawberry to antibiotics. Plant Growth Regul 65:183–193. https://doi.org/10.1007/s10725-011-9587-9
Queiroz EG, Degenhardt J, Quoirin M, Silva Kda (2020) Endophytic bacteria associated with tissue culture and leaves of Plinia Peruviana. Pesq Agropec Bras 55:e01844. https://doi.org/10.1590/S1678-3921.pab2020.v55.01844
Sant’Ana CR, de O, Paiva R, Reis MV et al (2018) dos, In vitro propagation of Campomanesia rufa: An endangered fruit species. Ciênc agrotec 42:372–380. https://doi.org/10.1590/1413-70542018424011018
Santoro MB, Brogio B, do A, Bueno SCS et al (2021) Propagação vegetativa de Campomanesia phaea Pelas técnicas de alporquia e enxertia| Santoro| pesquisa agropecuária Brasileira. Pesquisa Agropecuária Brasileira 56
Santoro MB, Brogio B, do A, Forte MJ et al (2022) Vegetative multiplication of the Atlantic rainforest species Eugenia involucrata. Pesq Agropec Bras 57:e02921. https://doi.org/10.1590/S1678-3921.pab2022.v57.02921
Santos HM, Lodeiro C, Capelo-Martínez J-L (2008) The power of ultrasound. In: Capelo-Martínez J-L. Ultrasound in chemistry. Wiley, Ltd, pp 1–16
de São José JFB, de Andrade NJ, Ramos AM et al (2014) Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 45:36–50. https://doi.org/10.1016/j.foodcont.2014.04.015
de Souza JA, Schuch MW, Donini LP, de Ribeiro M F (2008) Tipos e concentrações de citocinina Na multiplicação in vitro de Pitangueira. Cienc Rural 38:2046–2048. https://doi.org/10.1590/S0103-84782008000700040
Souza VC, Flores TB, Colletta GD, Coelho RLG (2018) Guia Das Plantas do Cerrado, 1st edn. Taxon, Piracicaba
Stefanel CM, Reiniger LRS, Silva LD et al (2020) da, Diodos emissores de luz (LEDS) no cultivo in vitro de Eugenia involucrata. Pesquisa Florestal Brasileira 40:. https://doi.org/10.4336/2020.pfb.40e201901930
Taver IB, Spricigo PC, Neto HB et al (2022) Bioactive compounds and in vitro antioxidant capacity of cambuci and Uvaia: an extensive description of Little-Known fruits from the myrtaceae family with high consumption potential. Foods 11:2612. https://doi.org/10.3390/foods11172612
Wilson PG (2011) Myrtaceae. In: Kubitzki K (ed) Flowering plants. Eudicots: sapindales, cucurbitales, myrtaceae. Springer, Berlin, Heidelberg, pp 212–271
Yildiz M, Fatih Ozcan S, Kahramanogullari T, Tuna C E (2012) The effect of sodium hypochlorite solutions on the viability and in vitro regeneration capacity of the tissue. NPJ 2:328–331. https://doi.org/10.2174/2210315511202040328
Author Information
Departamento de Produção Vegetal, Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, São Paulo, Brazil