Comparative pharmacognostical standardization of different parts of Euphorbia neriifolia Linn
Research Articles | Published: 15 November, 2022
First Page: 210
Last Page: 221
Views: 3793
Keywords: Euphorbia neriifolia , Fluorescence analysis, Heavy metals, Inorganic molecules, Pharmacognostical standardization, Phytochemical screening
Abstract
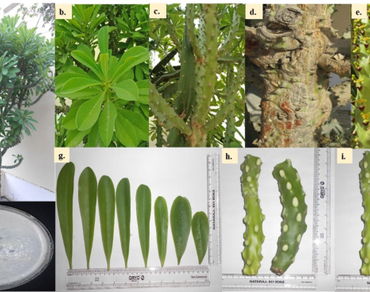
In the present study, determination of morphological characteristics, physicochemical analysis (proximate analysis, extractive value, screening of inorganic constituents, fluorescence analysis), and elemental analysis of various parts of Euphorbia neriifolia (EN) were carried out according to the guidelines of the World Health Organization (WHO). Qualitative phytochemical analysis was performed for the identification of bioactive constituents present in EN. Organoleptic characteristics found to be useful in evaluating the quality of EN parts and to classify them in terms of properties that influence the differential use of EN parts in treatment of diseases. Physicochemical analysis revealed the presence of lower content of total ash (0.315 ± 0.03; 0.335 ± 0.011 %w/w), water soluble ash (1.719 ± 0.190; 2.305 ± 0.08 %w/w), and acid insoluble ash (1.95 ± 0.045; 2.07 ± 0.05 %w/w) in the latex and leaf followed by other two parts. The sulfated ash value (0.065 ± 0.015; 0.067 ± 0.050 %w/w) was found to be lower in bark and leaf. The stem powder followed by other parts represented the lower content (10.8 ± 0.1 %d/w) of moisture. The value of pH ranged from 5.1 to 6.9 in the different part of EN. The maximum extractive value was reported in the aqueous extracts (12.794%) of EN stem. Among different inorganic constituents, phosphate and chloride were analysed in all the parts of EN. The fluorescence analysis showed characteristic change of color under different chemical treatments and heavy metal concentration (Cu, Mn, Cd, Pb, As, Zn, and Cr) was observed to be in permissible limit. The phytochemical screening (PS) revealed the presence of primary (carbohydrates, fixed oil and fats, and organic acids) and secondary (essential oil, alkaloids, flavonoids, polyphenols, terpenoids, and tannins) metabolites in high and moderate amount among all parts of EN. Thus, it can be concluded that the present study could serve as a base for the isolation and characterization of novel natural therapeutic agents for ethano-pharmacological research.
References
Akbar S, Hanif U, Ali J, Ishtiaq S (2014) Pharmacognostic studies of stem, roots and leaves of Malva parviflora L. Asian Pac J Trop Biomed 4(5):410–415. https://doi.org/10.12980/APJTB.4.2014C1107
Al Sulaibi MAM, Thiemann C, Thiemann T (2020) Chemical constituents and uses of. and Calotropis gigantea-A review Open Chem J 7:1–15. https://doi.org/10.2174/1874842202007010001
Alagar Raja M, Sushma K, Banji D, Rao KNV, Selvakumar D (2014) Evaluation of standardisation parameters, Pharmacognostic study, preliminary phytochemical screening and in vitro antidiabetic activity of Coccinia indica fruits as per WHO guidelines. Indian J Pharm Biol Res 2(3):54–64. https://doi.org/10.30750/ijpbr.2.3.9
Alam F, Us Saqib QN (2015) Pharmacognostic standardization and preliminary phytochemical studies of Gaultheria trichophylla. Pharm Biol 53(12):1711–1718. https://doi.org/10.3109/13880209.2014.1003355
Ali S, Khan MR, Irfanullah, Sajid M, Zahra Z (2018) Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement Altern Med 18:43. https://doi.org/10.1186/s12906-018-2114-z
Ankad GM, Pai SR, Upadhya V, Hurkadale PJ, Hegde HV (2015) Pharmacognostic evaluation of Achyranthes coynei: leaf. EJBAS 2(2015):25–31. https://doi.org/10.1016/j.ejbas.2014.12.002
Anonymous, Geneva (1998) https://www.who.int/docs/default-source/medicines/norms-and-standards/guidelines/quality-control/quality-control-methods-for-medicinal-plant-materials.pdf?sfvrsn=b451e7c6_0
Banothu V, Neelagiri C, Adepally U, Lingam J, Bommareddy K (2017) Phytochemical screening and evaluation of in vitro antioxidant and antimicrobial activities of the indigenous medicinal plants Albizia odoratissima. Pharm Biol 55(1):1155–1161. https://doi.org/10.1080/13880209.2017.1291694
Chase CR, Pratt RJ (1949) Florescence of powdered vegetable drugs with particular reference to development of systems of identification. J Am Pharm Assoc 38(6):324–331. https://doi.org/10.1002/jps.3030380612
Chaudhary P, Janmeda P (2021) Sehund: Poison or Medicine. Agriculture & Food: e-newsletter 3(4): 254–256. E-ISSN: 2581–8317.
Chaudhary P, Janmeda P (2022) Quantification of phytochemicals and in vitro antioxidant activities from various parts of Euphorbia neriifolia Linn. J Appl Biol Biotech 10(02):133–145. https://doi.org/10.7324/JABB.2022.100217
Dghaim R, Al Khatib S, Rasool H, Khan MA (2015) Determination of heavy metals concentration in traditional herbs commonly consumed in the United Arabs Emirates. J Environ Public Health 2015(4):1–6. https://doi.org/10.1155/2015/973878
Elendran S, Wang LW, Prankerd R, Palanisamy UD (2015) The physiochemical properties of geraniin, a potential antihyperglycemic agent. Pharm Biol 53(12):1719–1726. https://doi.org/10.3109/13880209.2014.1003356
Filipiak-Szok A, Kurzawa M, Cichosz M, Szlyk E (2015) Elemental analysis of medicinal herbs and dietary supplements. Anal Lett 48(16):2626–2638. https://doi.org/10.1080/00032719.2015.1041031.
Harborne JB (1993) Phytochemistry. Academic Press, London, pp 89–131
Ibrahim AM, Ghareeb MA (2020) Preliminary phytochemical screening, total phenolic content, In vitro antioxidant and molluscicidal activities of the methanolic extract of five medicinal plants on Biomphalaria alexandrina snails. J Herbs Spices Med Plants 26(1):40–48. https://doi.org/10.1080/10496475.2019.1666769
Karipiuk UV, Al Azzam KM, Abudayeh ZHM, Kislichenko V, Naddaf A, Cholak I, Yemelianova O (2016) Qualitative and Quantitative content determination of macro-minor elements in Bryonia alba L. roots using flame atomic absorption spectroscopy technique. Adv Pharm Bull 6(2):285–291. https://doi.org/10.15171/apb.2016.040
Khandelwal K (2007) Practical pharmacognosy, techniques and experiments. 17th edition. Nirali Prakashan, Pune, India
Kornerup A, Wanscher JH (1978) Methuen Handbook of Colour, 3rd edn. Eyre, London
Li J-C, Feng X-Y, Liu D, Zhang Z-J, Chen X-Q, Li R-T, Li H-M (2019) Diterpenoids from Euphorbia neriifolia and their related anti-HIV and cytotoxic activity. Chem Biodivers 16(12):e1900495. https://doi.org/10.1002/cbdv.201900495
Moazzem Hossen SM, Shahadat Hossain M, Yusuf ATM, Chaudhary P, Emon NU, Janmeda P (2021) Profiling of phytochemical and antioxidant activity of wild mushrooms: evidence from the in vitro study and phytoconstituent’s binding affinity to the human erythrocyte catalase and human glutathione reductase. Food Sci Nutr 10(1):88–102. https://doi.org/10.1002/fsn3.2650
Pandey D, Prasad SK, Begum AS, Hemalatha S, Wahi AK (2013) Pharmacognostical standardization and isolation of ergosterol from Melothria maderaspatana Linn. Int J Food Prop 16(8):1871–1882. https://doi.org/10.1080/10942912.2011.587624
Parveen R, Shamsi TN, Singh G, Athar T, Fatima S (2018) Phytochemical analysis and In-vitro bioschemical characterization of aqueous and methanolic extract of Triphala, a conventional herbal remedy. Biotechnol Rep 17:126–136. https://doi.org/10.1016/j.btre.2018.02.003
Sall ML, Diaw KKD, Gningue-Sall D, Aaron SE, Aaron J-J (2020) Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ Sci Pollut Res 27:29927–29942. https://doi.org/10.1007/a11356-020-09354-3
Sharma GK, Dhanawat M (2019) Effectual qualitative chemical evaluation of Euphorbia neriifolia Linn. by using fluorescence analysis. JDDT 9(1-S). https://doi.org/10.22270/jddt.v9i1-s.2248
Sharma V, Janmeda P (2014a) Protective assessment of Euphorbia neriifolia and its isolated flavonoid against N-nitrosodiethylamine-induced hepatic carcinogenesis in male mice: a histopathological analysis. Toxicol Int 21(1):37–43. https://doi.org/10.4103/0971-6580.128790
Sharma V, Janmeda P (2014b) Extraction, isolation, and identification of flavonoid from Euphorbia neriifolia leaves. Arab J Chem 10:509–514. https://doi.org/10.1016/j.arabjc.2014.08.019
Sharma V, Prachaeta (2013) Microscopic studies and preliminary pharmacognostical evaluation of Euphorbia neriifolia L. leaves. Indian J Nat Prod Resour 4(4):348–357
World Health Organization (2007) WHO guidelines for assessing quality of herbal medicines to contaminants and residues. World Health Organization, Geneva. https://apps.who.int/iris/handle/10665/43510
Author Information
Department of Bioscience and Biotechnology, Banasthali Vidyapith, Rajasthan, India