Antioxidant potential and impact of different extraction solvents on the free, esterified and insoluble-bound phenolics, flavonoid and tannin content of Trillium govanianum Wall ex D. Don, a rare Himalayan herb
Chandola Vaishali, Chandra Sudeep, Nautiyal A. R., Concenço Germani
Research Articles | Published: 07 April, 2022
First Page: 953
Last Page: 960
Views: 3640
Keywords: Trillium , Bioactive yield, Antioxidant, FRAP, Phenolic content, Flavonoid
Abstract
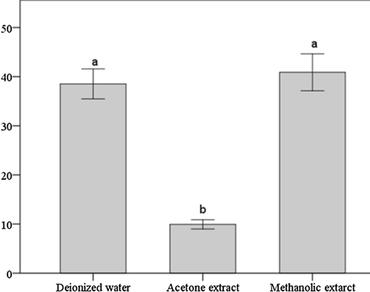
Trillium govanianum is a compelling medicinal herb that mainly occurs in the sub-alpine and alpine regions of the Himalayan states of India. It has acquired a threatened status due to large-scale illicit exploitation because of its effective medicinal properties. A limited number of studies on the antioxidant potential of T. govanianum along with its establishment as a source of new antioxidants led us to carry out this investigation. The study also investigated the efficacy of three different solvents on the quantitative extraction of bioactive compounds from the herb for its maximum utilization. For the study, rhizomes of T. govanianum were subjected to enzymatic and non-enzymatic assays. Analysis of enzymes catalase (CAT) and ascorbate peroxidase (AP) was carried out by simple phosphate buffer extraction method, phenolics and tannin determination by Folin–Ciocalteu method, flavonoid content by aluminium chloride colorimetric method and also total reducing and ferric reducing power assays were done. Results showed that rhizomes of T. govanianum exhibit fair CAT and AP activity. From the results it was also apparent that absolute methanol was the most feasible solvent among the all three used, to get the highest solid extractable yield (40.24%) as well as total phenolics and tannins (46.7 ± 0.15 and 0.74 ± 0.01 mg/gm of extract respectively) whereas acetone extract yielded more flavonoid content (16.5 ± 0.15 mg/gm of extract). FRAP activity and total reducing power of the methanol extract was also higher than the other two extracts. These findings reveal a promising potential of this herb as a source of antioxidants comprehending a need for its cultivation and conservation.
References
Acosta-Estrada BA, Gutierrez-Uribe JA, Serna-Saldivar SO (2014) Bound phenolics in foods, a review. Food Chem 152:46–55. https://doi.org/10.1016/j.foodchem.2013.11.093
Aebi H (1983) Catalase in vitro. Methods Enzymol 105:121–126
Agbo MO, Uzorl PF, Akazie-Nneji UN, Eze-Odurukwe CU, Ogbatue UB, Mbaoji EC (2015) Antioxidant, total phenolic and flavonoid content of selected Nigerian medicinal plants. Dhaka Univ J Pharm Sci 14(1):35–41
Arruda HS, Pereira GA, de Morais DR, Eberlin MN, Pastore GM (2018) Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit ( Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem 245:738–749. https://doi.org/10.1016/j.foodchem.2017.11.120
Benzie IFF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Chandra S, Singh A, Singh CP, Nautiyal MC, Rawat LS (2018) Vascular plants distribution in relation to topography and environmental variables in the alpine zone of Kedarnath Wild Life Sanctuary, West Himalaya. J Mt Sci 15(9):1936–1949
Chandran KCI, Indira G (2016) Quantitative estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of Strobilanthes Kunthiana (Neelakurinji). J Med Plants Stud 4(4):282–286
Chauhan NS (1999) Medicinal and aromatic plants of Himachal Pradesh. Indus Publishing Company, New Delhi
Cheynier V (2012) Phenolic compounds: from plants to foods. Phytochem Rev 11:153–177. https://doi.org/10.1007/s11101-012-9242-8
Corpas FJ, Barroso JB, del Río LA (2001) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci 6:145–150
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15(10):7313–7352
D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R (2007) Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita 43(4):348–361
Das S, Talukdar D, Sangha MK, Chaudhary DP, Borah N, Das A, Das S, Saikia SP (2017) Antioxidant enzymes potential in leaves of oats and barley and phytochemistry of stress tolerance. J Pharmacogn Phytochem 1:694–703
Do Diem Q, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju Y-H (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic. J Food Drug Anal 22(3):296–302
Ezez D, Tefera M (2021) Effects of solvents on total phenolic content and antioxidant activity of ginger extracts. J Chem. https://doi.org/10.1155/2021/6635199
Ghasemzadeh A, Jaafar Hawa ZE, Rahmat A (2010) Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 15:4324–4333
Haminiuk CWI, Plata-Oviedo MSV, de Mattos G, Carpes ST, Branco IG (2014) Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J Food Sci Technol 51(10):2862–2866
Kenganora M, Bhaskaran M, Santhepete MN, Kumar V, Hukkeri I (2017) Antioxidant potential of a toxic plant Calotropis procera R. Br. Free Radic Antioxid 7(2):143–151
Krygier K, Sosulski F, Hodge L (1982) Free, esterified, and insoluble bound phenolic acids. 1. Extraction and purification procedure. J Agric Food Chem 30:330–334
Liyana-Pathirana CM, Shahidi F (2006) Importance of insoluble-bound phenolics to antioxidant properties of wheat. J Agric Food Chem 54(4):1256–1264. https://doi.org/10.1021/jf052556h
Mahmood A, Mahmood A, Malik RN (2012) Indigenous knowledge of medicinal plants from Leepa valley, Azad Jammu and Kashmir, Pakistan. J Ethnopharmacol 143(1):338–346
Medini F, Fellah H, Ksouri R, Abdelly C (2014) Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J Taibah Univ Sci 8:216–224
Mueller-Harvey I (2006) Unraveling the conundrum of tannins in animal nutrition and health. J Sci Food Agric 86:2010–2037
Naczk M, Shahidi F (1989) The effect of methanol-ammonia-water treatment on the content of phenolic acids of canola. Food Chem 31:159–164
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ngo TV, Scarlett CJ, Bowyer MC, Ngo PD, Vuong QV (2017) Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J Food Qual 1:1–8
Nguyen NVT, Duong NT, Nguyen KNH, Bui NT, Pham TLT, Nguyen KT, Le PH, Kim KH (2021) Effect of extraction solvent on total phenol, flavonoid content, and antioxidant activity of Avicennia officinalis. Biointerface Res Appl Chem 12(2):2678–2690
Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315
Pant S, Samant S (2010) Ethnobotanical observations in the Mornaula reserve forest of Kumaun, West Himalaya, India. Ethnobot Leaflets 14:193–217
Rahman SU, Ismail M, Shah MR, Iriti M, Shahid M (2015) GC/MS analysis, free radical scavenging, anticancer and β-glucuronidase inhibitory activities of Trillium govanianum rhizome. Bangladesh J Pharmacol 10:577–583
Rahman SU, Adhikari A, Ismail M, Shah MR, Khurram M, Shahid M, Ali F, Haseeb A, Akbar F, Iriti M (2016) Beneficial effects of Trillium govanianum rhizomes in pain and inflammation. Molecules 21:1095
Samant SS, Dhar U, Palni LMS (1998) Medicinal plants of indian himalaya: diversity, distribution potential values. Gyanodaya Prakashan, Nainital
Shahidi F, Ju-Dong Y (2016) Insoluble-bound phenolics in food. Molecules 21:1216. https://doi.org/10.3390/molecules21091216
Singleton V, Rossi J (1965) Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Truong DH, Nguyen DH, Ta Anh NT, Vo Bui A, Ha Do T, Nguyen HC (2019) Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J Food Q. https://doi.org/10.1155/2019/8178294
Widyawati PS, Budianta TDW, Kusuma FA, Wijaya EL (2014) Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indica less leaves extracts. Int Pharmacol Phytochem Res 6(4):850–855
Author Information
High Altitude Plant Physiology Research Center, H. N. B. Garhwal University, Garhwal, India