Mechanism and molecular basis of apomixis in angiosperms
*Article not assigned to an issue yet
Review Articles | Published: 20 April, 2024
Online ISSN : 2229-4473.
Website:www.vegetosindia.org
Pub Email: contact@vegetosindia.org
First Page: 0
Last Page: 0
Views: 226
Keywords:
Apomixis, Apomeiosis, Clonal seeds, Heterosis, Parthenogenesis
Abstract
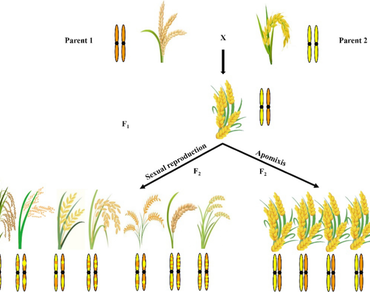
Hybrids with high yield and stress tolerance have been developed by researchers and cultivated by farmers over the years. However, their genetic constitution will change in the segregating generations and the elite traits of the hybrids will not be retained completely in the subsequent generations. These limitations can be overcome by exploiting the asexual mode of reproduction that exists in flowering plants known as apomixis. This phenomenon has the potential to produce clonal or genetically identical seeds as the mother genotype and it can also preserve the hybrid vigour for several generations. In angiosperms, it is essential to understand the mechanism and molecular basis of apomixis to transfer or convert non-apomictic plants to apomictic plants. Different genes/alleles or genetic mechanisms controlling various steps of apomixis have already been reported. Recently, few studies were reported on how non-apomictic plants can be converted into apomictic lines by using two approaches viz., hybridization and CRISPR/Cas gene editing method, to develop synthetic apomictic lines. This synthetic apomixis brought an initial breakthrough by producing clonal hybrid seeds (> 90 per cent) over multiple generations. This review highlights the mechanisms, molecular basis of apomixis and recent developments, achievements and challenges in apomixis, as a tool for clonal seed production, preservation of heterosis and crop improvement.
(*Only SPR Members can get full access. Click Here to Apply and get access)
References
Albertini E, Barcaccia G, Mazzucato A et al (2010) Apomixis in the Era of Biotechnology. In: Pua EC, Davey MR (eds) Plant Developmental Biology - Biotechnological Perspectives. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 405–436
Bai X, Peirson BN, Dong F et al (1999) Isolation and characterization of SYN1, a RAD21 -like gene essential for meiosis in arabidopsis. Plant Cell 11:417–430. https://doi.org/10.1105/tpc.11.3.417
Benyahya F, Nadaud I, Da Ines O et al (2020) SPO11.2 is essential for programmed double-strand break formation during meiosis in bread wheat (Triticumaestivum L.). Plant J 104:30–43. https://doi.org/10.1111/tpj.14903
Berger F, Hamamura Y, Ingouff M, Higashiyama T (2008) Double fertilization:caught in the act. Trends Plant Sci 13:437–443. https://doi.org/10.1016/j.tplants.2008.05.011
Bhatt AM, Lister C, Page T et al (1999) The DIF1 gene of Arabidopsis is required for meiotic chromosome segregation and belongs to the REC8/RAD21 cohesin gene family. Plant J 19:463–472. https://doi.org/10.1046/j.1365-313X.1999.00548.x
Boutilier K, Offringa R, Sharma VK et al (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749. https://doi.org/10.1105/tpc.001941
Cai X, Dong F, Edelmann RE, Makaroff CA (2003) The Arabidopsis SYN1 cohesin protein is required for sister chromatid arm cohesion and homologous chromosome pairing. J Cell Sci 116:2999–3007. https://doi.org/10.1242/jcs.00601
Chelysheva L, Diallo S, Vezon D et al (2005) AtREC8 and AtSCC3 are essential to the monopolar orientation of the kinetochores during meiosis. J Cell Sci 118:4621–4632. https://doi.org/10.1242/jcs.02583
Conner JA, Ozias-Akins P (2017) Apomixis: Engineering the Ability to Harness Hybrid Vigor in Crop Plants. In: Schmidt A (ed) Plant Germline Development. Springer, New York, New York, NY, pp 17–34
Cromer L, Heyman J, Touati S et al (2012) OSD1 Promotes meiotic progression via APC/C inhibition and forms a regulatory network with TDM and CYCA1;2/TAM. PLoS Genet 8:e1002865. https://doi.org/10.1371/journal.pgen.1002865
d’Erfurth I, Jolivet S, Froger N et al (2009) Turning meiosis mitosis. Plosbiol 7:e1000124. https://doi.org/10.1371/journal.pbio.1000124
d’Erfurth I, Cromer L, Jolivet S et al (2010) The CYCLIN-A CYCA1;2/TAM Is required for the meiosis I to meiosis II transition and cooperates with OSD1 for the prophase to first meiotic division transition. PLoS Genet 6:e1000989. https://doi.org/10.1371/journal.pgen.1000989
De Muyt A, Vezon D, Gendrot G et al (2007) AtPRD1 is required for meiotic double strand break formation in arabidopsis thaliana. EMBO J 26:4126–4137. https://doi.org/10.1038/sj.emboj.7601815
De Muyt A, Pereira L, Vezon D et al (2009) A high throughput genetic screen identifies new early meiotic recombination functions in arabidopsis thaliana. PLoS Genet 5:e1000654. https://doi.org/10.1371/journal.pgen.1000654
Demirer GS, Zhang H, Matos JL et al (2019) High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat Nanotechnol 14:456–464. https://doi.org/10.1038/s41565-019-0382-5
Dong F, Cai X, Makaroff CA (2001) Cloning and characterization of two Arabidopsis genes that belong to the RAD21/REC8 family of chromosome cohesin proteins. Gene 271:99–108. https://doi.org/10.1016/S0378-1119(01)00499-1
Fayos I, Meunier AC, Vernet A et al (2020) Assessment of the roles of SPO11-2 and SPO11-4 in meiosis in rice using CRISPR/Cas9 mutagenesis. J Exp Bot 71:7046–7058. https://doi.org/10.1093/jxb/eraa391
Fiaz S, Wang X, Younas A et al (2021) Apomixis and strategies to induce apomixis to preserve hybrid vigor for multiple generations. GM Crops & Food 12:57–70. https://doi.org/10.1080/21645698.2020.1808423
Gilles LM, Khaled A, Laffaire J et al (2017) Loss of pollen-specific phospholipase NOT LIKE DAD triggers gynogenesis in maize. EMBO J 36:707–717. https://doi.org/10.15252/embj.201796603
Golubovskaya IN, Hamant O, Timofejeva L et al (2006) Alleles of afd1 dissect REC8 functions during meiotic prophase I. J Cell Sci 119:3306–3315. https://doi.org/10.1242/jcs.03054
Grelon M (2001) AtSPO11-1 is necessary for efficient meiotic recombination in plants. EMBO J 20:589–600. https://doi.org/10.1093/emboj/20.3.589
Gruber S, Haering CH, Nasmyth K (2003) Chromosomal cohesin forms a ring. Cell 112:765–777. https://doi.org/10.1016/S0092-8674(03)00162-4
Gualtieri G, Conner JA, Morishige DT et al (2006) A Segment of the apospory-specific genomic region is highly microsyntenic not only between the apomicts pennisetumsquamulatum and buffelgrass, but also with a rice chromosome 11 centromeric-proximal genomic Region. Plant Physiol 140:963–971. https://doi.org/10.1104/pp.105.073809
Hamant O, Golubovskaya I, Meeley R et al (2005) A REC8-dependent plant shugoshin is required for maintenance of centromeric cohesion during meiosis and has no mitotic functions. Curr Biol 15:948–954. https://doi.org/10.1016/j.cub.2005.04.049
Hand ML, Koltunow AMG (2014) The genetic control of apomixis: asexual seed formation. Genetics 197:441–450. https://doi.org/10.1534/genetics.114.163105
Harashima H, Dissmeyer N, Schnittger A (2013) Cell cycle control across the eukaryotic kingdom. Trends Cell Biol 23:345–356. https://doi.org/10.1016/j.tcb.2013.03.002
Hartung F, Wurz-Wildersinn R, Fuchs J et al (2007) The Catalytically Active Tyrosine Residues of Both SPO11-1 and SPO11-2 Are required for meiotic double-strand break induction in arabidopsis. Plant Cell 19:3090–3099. https://doi.org/10.1105/tpc.107.054817
Hochholdinger F, Baldauf JA (2018) Heterosis in plants. Curr Biol 28:R1089–R1092. https://doi.org/10.1016/j.cub.2018.06.041
Jiang C, Sun J, Li R et al (2022) A reactive oxygen species burst causes haploid induction in maize. Mol Plant 15:943–955. https://doi.org/10.1016/j.molp.2022.04.001
Jing J-L, Zhang T, Kao Y-H et al (2020) ZmMTOPVIB enables DNA double-strand break formation and bipolar spindle assembly during maize meiosis. Plant Physiol 184:1811–1822. https://doi.org/10.1104/pp.20.00933
Keeney S, Giroux CN, Kleckner N (1997) Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88:375–384. https://doi.org/10.1016/S0092-8674(00)81876-0
Kelliher T, Starr D, Richbourg L et al (2017) MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 542:105–109. https://doi.org/10.1038/nature20827
Kelliher T, Starr D, Su X et al (2019) One-step genome editing of elite crop germplasm during haploid induction. Nat Biotechnol 37:287–292. https://doi.org/10.1038/s41587-019-0038-x
Khanday I, Skinner D, Yang B et al (2019) A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 565:91–95. https://doi.org/10.1038/s41586-018-0785-8
Koltunow AM (1993) Apomixis: embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell. https://doi.org/10.1105/tpc.5.10.1425
Ku J-C, Ronceret A, Golubovskaya I et al (2020) Dynamic localization of SPO11-1 and conformational changes of meiotic axial elements during recombination initiation of maize meiosis. PLoS Genet 16:e1007881. https://doi.org/10.1371/journal.pgen.1007881
León-Martínez G, Vielle-Calzada J-P (2019) Apomixis in flowering plants: developmental and evolutionary considerations. Current Topics in Developmental Biology. Elsevier, pp 565–604
Li X, Meng D, Chen S et al (2017) Single nucleus sequencing reveals spermatid chromosome fragmentation as a possible cause of maize haploid induction. Nat Commun 8:991. https://doi.org/10.1038/s41467-017-00969-8
Liu C, Li X, Meng D et al (2017) A 4-bp Insertion at ZmPLA1 encoding a putative phospholipase a generates haploid induction in maize. Mol Plant 10:520–522. https://doi.org/10.1016/j.molp.2017.01.011
Liu H, Wang K, Jia Z et al (2020) Efficient induction of haploid plants in wheat by editing of TaMTL using an optimized agrobacterium-mediated CRISPR system. J Exp Bot 71:1337–1349. https://doi.org/10.1093/jxb/erz529
Liu C, He Z, Zhang Y et al (2023) Synthetic apomixis enables stable transgenerational transmission of heterotic phenotypes in hybrid rice. Plant Commun 4:100470. https://doi.org/10.1016/j.xplc.2022.100470
Mahlandt A, Singh DK, Mercier R (2023) Engineering Apomixis Crops. Theorappl Genet 136:131. https://doi.org/10.1007/s00122-023-04357-3
Marston AL, Amon A (2004) Meiosis: cell-cycle controls shuffle and deal. Nat Rev Mol Cell Biol 5:983–997. https://doi.org/10.1038/nrm1526
Miao C, Tang D, Zhang H et al (2013) CENTRAL REGION COMPONENT1, a Novel Synaptonemal Complex Component, Is Essential for Meiotic Recombination Initiation in Rice. Plant Cell 25:2998–3009. https://doi.org/10.1105/tpc.113.113175
Mieulet D, Jolivet S, Rivard M et al (2016) Turning rice meiosis into mitosis. Cell Res 26:1242–1254. https://doi.org/10.1038/cr.2016.117
Nasmyth K (2002) Segregating sister genomes: the molecular biology of chromosome separation. Science 297:559–565. https://doi.org/10.1126/science.1074757
Nonomura K-I, Nakano M, Fukuda T et al (2004) The Novel Gene HOMOLOGOUS PAIRING ABERRATION IN RICE MEIOSIS1 of rice encodes a putative coiled-coil protein required for homologous chromosome pairing in meiosis. Plant Cell 16:1008–1020. https://doi.org/10.1105/tpc.020701
Ozias-Akins P, Conner JA (2020) Clonal reproduction through seeds in sight for crops. Trends Genet 36:215–226. https://doi.org/10.1016/j.tig.2019.12.006
Pawlowski WP, Wang C-JR, Golubovskaya IN et al (2009) Maize AMEIOTIC1 is essential for multiple early meiotic processes and likely required for the initiation of meiosis. ProcNatlAcadSci USA 106:3603–3608. https://doi.org/10.1073/pnas.0810115106
Pesin JA, Orr-Weaver TL (2008) Regulation of APC/C Activators in mitosis and meiosis. Annu Rev Cell DevBiol 24:475–499. https://doi.org/10.1146/annurev.cellbio.041408.115949
Ravi M, Chan SWL (2010) Haploid plants produced by centromere-mediated genome elimination. Nature 464:615–618. https://doi.org/10.1038/nature08842
Ravi M, Marimuthu MPA, Siddiqi I (2008) Gamete formation without meiosis in arabidopsis. Nature 451:1121–1124. https://doi.org/10.1038/nature06557
Sailer C, Schmid B, Grossniklaus U (2016) Apomixis allows the transgenerational fixation of phenotypes in hybrid plants. Curr Biol 26:331–337. https://doi.org/10.1016/j.cub.2015.12.045
Shao T, Tang D, Wang K et al (2011) OsREC8 is essential for chromatid cohesion and metaphase i monopolar orientation in rice meiosis. Plant Physiol 156:1386–1396. https://doi.org/10.1104/pp.111.177428
Shi W, Ji J, Xue Z et al (2021) PRD1, a homologous recombination initiation factor, is involved in spindle assembly in rice meiosis. New Phytol 230:585–600. https://doi.org/10.1111/nph.17178
Siddiqi I, Ganesh G, Grossniklaus U, Subbiah V (2000) The dyad gene is required for progression through female meiosis in Arabidopsis. Development 127:197–207. https://doi.org/10.1242/dev.127.1.197
Stacey NJ, Kuromori T, Azumi Y et al (2006) Arabidopsis SPO11-2 functions with SPO11-1 in meiotic recombination. Plant J 48:206–216. https://doi.org/10.1111/j.1365-313X.2006.02867.x
Steckenborn S, Cuacos M, Ayoub MA et al (2023) The meiotic topoisomerase VI B subunit (MTOPVIB) is essential for meiotic DNA double-strand break formation in barley (Hordeumvulgare L.). Plant Reprod 36:1–15. https://doi.org/10.1007/s00497-022-00444-5
Sun G, Geng S, Zhang H et al (2022) Matrilineal empowers wheat pollen with haploid induction potency by triggering postmitosis reactive oxygen species activity. New Phytol 233:2405–2414. https://doi.org/10.1111/nph.17963
Tang Y, Yin Z, Zeng Y et al (2017) MTOPVIB interacts with AtPRD1 and plays important roles in formation of meiotic DNA double-strand breaks in arabidopsis. Sci Rep 7:10007. https://doi.org/10.1038/s41598-017-10270-9
Underwood CJ, Mercier R (2022) Engineering apomixis: clonal seeds approaching the fields. Annu Rev Plant Biol 73:201–225. https://doi.org/10.1146/annurev-arplant-102720-013958
Underwood CJ, Vijverberg K, Rigola D et al (2022) A PARTHENOGENESIS allele from apomictic dandelion can induce egg cell division without fertilization in lettuce. Nat Genet 54:84–93. https://doi.org/10.1038/s41588-021-00984-y
Vernet A, Meynard D, Lian Q et al (2022) High-frequency synthetic apomixis in hybrid rice. Nat Commun 13:7963. https://doi.org/10.1038/s41467-022-35679-3
Vrielynck N, Chambon A, Vezon D et al (2016) A DNA topoisomerase VI–like complex initiates meiotic recombination. Science 351:939–943. https://doi.org/10.1126/science.aad5196
Xiong J, Hu F, Ren J et al (2023) Synthetic apomixis: the beginning of a new era. Curr Opin Biotechnol 79:102877. https://doi.org/10.1016/j.copbio.2022.102877
Xue Z, Li Y, Zhang L et al (2016) OsMTOPVIB promotes meiotic DNA double-strand break formation in rice. Mol Plant 9:1535–1538. https://doi.org/10.1016/j.molp.2016.07.005
Xue Z, Liu C, Shi W et al (2019) OsMTOPVIB is required for meiotic bipolar spindle assembly. Proc Natl Acad Sci USA 116:15967–15972. https://doi.org/10.1073/pnas.1821315116
Yao L, Zhang Y, Liu C et al (2018) OsMATL mutation induces haploid seed formation in indica rice. Nature Plants 4:530–533. https://doi.org/10.1038/s41477-018-0193-y
Yu H-G, Dawe RK (2000) Functional redundancy in the maize meiotic kinetochore. J Cell Biol 151:131–142. https://doi.org/10.1083/jcb.151.1.131
Yu H, Wang M, Tang D et al (2010) OsSPO11-1 is essential for both homologous chromosome pairing and crossover formation in rice. Chromosoma 119:625–636. https://doi.org/10.1007/s00412-010-0284-7
Zamariola L, De Storme N, TiangCl, et al (2013) SGO1 but not SGO2 is required for maintenance of centromere cohesion in arabidopsis thaliana meiosis. Plant Reprod 26:197–208. https://doi.org/10.1007/s00497-013-0231-x
Zhang L, Tao J, Wang S et al (2006) The Rice OsRad21-4, an orthologue of yeast Rec8 protein, is required for efficient meiosis. Plant MolBiol 60:533–554. https://doi.org/10.1007/s11103-005-4922-z
Zhang C, Song Y, Cheng Z et al (2012) The Arabidopsis thaliana DSB formation ( AtDFO ) gene is required for meiotic double-strand break formation: AtDFO is required for DSB formation. Plant J 72:271–281. https://doi.org/10.1111/j.1365-313X.2012.05075.x
Zhong Y, Liu C, Qi X et al (2019) Mutation of ZmDMP enhances haploid induction in maize. Nat Plants 5:575–580. https://doi.org/10.1038/s41477-019-0443-7
Zhong Y, Chen B, Li M et al (2020) A DMP-triggered in vivo maternal haploid induction system in the dicotyledonous arabidopsis. Nat Plants 6:466–472. https://doi.org/10.1038/s41477-020-0658-7
Acknowledgements
Author Information
Department of Plant Breeding and Genetics, Kerala Agricultural University, Thrissur, India
Department of Plant Breeding and Genetics, Kerala Agricultural University, Thrissur, India
jiji.joseph@kau.in
Department of Plant Breeding and Genetics, Kerala Agricultural University, Thrissur, India