UV-C and gamma radiation mediated L-DOPA production from in-vitro cultures of Mucuna pruriens (L.) DC
Bhaskar Rakesh, Nagella Praveen, Madhu A, Suriyamurthy N, Srinatha N
Research Articles | Published: 27 February, 2024
First Page: 721
Last Page: 731
Views: 3208
Keywords: Gamma radiation, UV-C radiation, In-vitro elicitation, n Mucuna pruriensn , L-DOPA, Cell cultures
Abstract
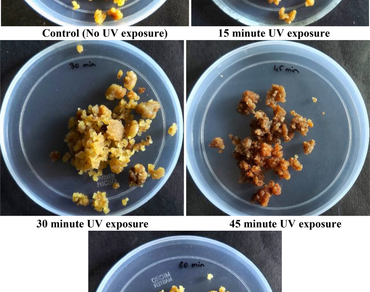
This is the first report on UV-C and gamma rays mediated in-vitro elicitation of L-DOPA from Mucuna pruriens (L.) DC. cell suspension cultures. Gamma and ultraviolet rays are used on plants to induce mutations which results in activation of defence cascades and production of secondary metabolites due to this abiotic stress. The in-vitro callus developed from 0.5 mg/L picloram was suspended into liquid medium and exposed to different time intervals (0, 15, 30, 45 and 60 min) of UV-C radiations. On the other hand, the seeds were directly exposed to different doses (25, 50, 100, 150 and 200 Gy) of gamma radiations and these irradiated seeds were grown in-vitro from which callus and cell cultures were established. From all these in-vitro cultures, the anti-Parkinson’s drug L-DOPA was quantified using HPLC. 60 and 30-minute exposure of UV-C radiations resulted in highest biomass (193.27 g/L FW) and L-DOPA production (5.13 mg/g DW) respectively both showing a 1.5-fold increase than the control. In gamma radiation studies, 100 Gy (Gy) dose showed the highest (83%) seed germination rate, 150 Gy increased the in-vitro root and shoot length, while 100 Gy increased the biomass of the cell cultures. Also, 150 Gy dose showed a 6.1, 2.6 and 2.4-fold increase in L-DOPA production in the in-vitro roots, in-vitro shoots, and cell suspension culture respectively when compared to the control. UV light exposure of 30 min and 150 Gy doses of gamma radiation showed a significant increase in L-DOPA production.
References
Agarwal H, Menon S, Shanmugam VK (2020) Functionalization of zinc oxide nanoparticles using Mucuna pruriens and its antibacterial activity. Surf Interfaces 19(April):100521
Alam N, Anis M, Javed S, Bin, Alatar AA (2020) Stimulatory effect of copper and zinc sulphate on plant regeneration, glutathione-S-transferase analysis and assessment of antioxidant activities in Mucuna pruriens L. (DC). Plant Cell Tiss Org Cult 141(1):155–166
Aly AA, El-Beltagi HES (2010) Influence of ionizing irradiation on the antioxidant enzymes of Vicia faba L. Grasas Aceites 61(3):288–294
Azeez H, Ibrahim K, Pop R, Pamfil D, Hârţa M, Bobiș O (2017) Changes induced by gamma ray irradiation on biomass production and secondary metabolites accumulation in Hypericum Triquetrifolium Turra callus cultures. Ind Crops Prod 108(February):183–189
Bertoli A, Lucchesini M, Mensuali-Sodi A, Leonardi M, Doveri S, Magnabosco A, Pistelli L (2013) Aroma characterisation and UV elicitation of purple basil from different plant tissue cultures. Food Chem 141(2):776–787. https://doi.org/10.1016/j.foodchem.2013.02.081
Bhaskar R, Xavier LSE, Udayakumaran G, Kumar DS, Venkatesh R, Nagella P (2021) Biotic elicitors: a boon for the in-vitro production of plant secondary metabolites. Plant Cell Tiss Org Cult. 1–18
Bhat R, Sridhar KR, Tomita-Yokotani K (2007) Effect of ionizing radiation on antinutritional features of velvet bean seeds (Mucuna pruriens). Food Chem 103:860–866
Chakravarty B, Sen (2001) Enhancement of regeneration potential and variability by γ-irradiation in cultured cells of Scilla indica. Biol Plant 44(4):189–193
Charbaji T, Nabulsi I (1999) Effect of low doses of gamma irradiation on in vitro growth of grapevine. Plant Cell Tiss Org Cult 57(2):129–132
Chattopadhyay S, Datta SK, Mahato SB (1995) Rapid micropropagation for Mucuna pruriens f. pruriens L. Plant Cell Rep 15:271–273
Chung BY, Lee YB, Baek MH, Kim JH, Wi SG, Kim JS (2006) Effects of low-dose gamma-irradiation on production of shikonin derivatives in callus cultures of Lithospermum erythrorhizon S. Radiat Phys Chem 75(9):1018–1023
Darakhshanda N, Tabasum T, Mahmooduzaffar SAH, Subhan S (2014) Radiation sensitivity of Cajanus cajan to gamma radiations. J Food Process Technol 5(12)
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7(7):1085–1097
El-aal MSA, Rabie KAE, Manaf HH (2016) The Effect of Uv-C on secondary metabolites production of Echinacea purpurea culture in vitro. J Biol Chem Environ Sci 11(2):465–483
El-Beltagi HS, Ahmed OK, El-Desouky W (2011) Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of Rosemary (Rosmarinus officinalis L.) callus culture. Radiat Phys Chem 80(9):968–976
Elsayed T, Ahamed S (2019) Bioprospecting elicitation with gammairradiation combine with chitosan to enhance, yield production, bioactive secondary metabolites and antioxidant activity for Saffron. J Plant Sci 7(6):137–143
Faisal M, Siddique I, Anis M (2006) An efficient plant regeneration system for Mucuna pruriens L. (DC.) Using cotyledonary node explants. Vitr Cell Dev Biol - Plant 42(1):59–64
Freitas A, Moldão-Martins M, Costa HS, Albuquerque TG, Valente A, Sanches-Silva A (2015) Effect of UV-C radiation on bioactive compounds of pineapple (Ananas comosus L. Merr.) By-products. J Sci Food Agric 95(1):44–52
Garcia MXU, Foote C, Van Es S, Devreotes PN, Alexander S, Alexander H (2000) Differential developmental expression and cell type specificity of Dictyostelium catalases and their response to oxidative stress and UV-light. Biochim Biophys Acta - Gene Struct Expr 1492:295–310
Gil M, Pontin M, Berli F, Bottini R, Piccoli P (2012) Metabolism of terpenes in the response of grape (Vitis vinifera L.) leaf tissues to UV-B radiation. Phytochemistry [Internet] 77:89–98. https://doi.org/10.1016/j.phytochem.2011.12.011
Halder M, Sarkar S, Jha S (2019) Elicitation: a biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci 19(12):880–895
Horling F, Lamkemeyer P, Ko J, Finkemeier I, Kandlbinder A, Baier M, Dietz K (2003) Dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiol 131(January):317–325
Janarthanam B, Sumathi E (2015) Optimization of biomass culture yield and L-dopa compound in the callus culture from cotyledonary leaves of Mucuna pruriens. Asian J Pharm Clin Res 8(4):282–286
Jones AMP, Saxena PK (2013) Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: a novel approach to reduce oxidative browning in plant tissue culture. PLoS ONE, 8(10), e76802
Kala BK, Mohan VR (2012) Effect of microwave treatment on the antinutritional factors of two accessions of velvet bean, Mucuna pruriens (L.) DC. Var. Utilis (wall. Ex wight) bak. Ex Burck. Int Food Res J 19(3):961–969
Khalil SA, Ahmad N, Zamir R (2015) Gamma radiation induced variation in growth characteristics and production of bioactive compounds during callogenesis in Stevia rebaudiana. New Negatives Plant Sci 1–2:1–5
Lahiri K, Mukhopadhyay MJ, Desjardins Y, Mukhopadhyay S (2012) Rapid and stable in vitro regeneration of plants through callus morphogenesis in two varieties of Mucuna pruriens L. - An Anti-parkinson’s drug yielding plant. Nucl 55(1):37–43
Lahiri K, Mukhopadhyay MJ, Mukhopadhyay S (2011) Enhancement of L-DOPA production in micropropagated plants of two different varieties of Mucuna pruriens L., available in India. Plant Tissue Cult Biotechnol 21(2):115–125
Lampariello LR, Cortelazzo A, Guerranti R, Sticozzi C, Valacchi G (2011) The magic velvet bean of Mucuna pruriens. J Tradit Complement Med 2(4):331–339
Li D, Luo Z, Mou W, Wang Y, Ying T, Mao L (2014) ABA and UV-C effects on quality, antioxidant capacity and anthocyanin contents of strawberry fruit (Fragaria Ananassa Duch). Postharvest Biol Technol 90:56–62. https://doi.org/10.1016/j.postharvbio.2013.12.006
Liu R, Xu S, Li J, Hu Y, Lin Z (2006) Expression profile of a PAL gene from Astragalus membranaceus var. Mongholicus and its crucial role in flux into flavonoid biosynthesis. Plant Cell Rep 25(7):705–710
Luthra PM, Singh S (2010) Identification and optimization of tyrosine hydroxylase activity in Mucuna pruriens DC. Var. Utilis. Planta 231(6):1361–1369
Martínez-Silvestre KE, Santiz-Gómez JA, Luján-Hidalgo MC, Ruiz-Lau N, Sánchez-Roque Y, Gutiérrez-Miceli FA (2022) Effect of UV-B radiation on flavonoids and phenols accumulation in tempisque (Sideroxylon Capiri Pittier) callus. Plants 11(4):473
Misra L, Wagner H (2004) Alkaloidal constituents of Mucuna pruriens seeds. Phytochemistry 65:2565–2567
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:474–497
Nabi N, Singh S, Saffeullah P (2021) Responses of in vitro cell cultures to elicitation: regulatory role of jasmonic acid and methyl jasmonate: a review. Vitr Cell Dev Biol - Plant 57(3):341–355
Norfadzrin F, Ahmed OH, Shaharudin S, Abdul Rahman D (2007) A preliminary study on gamma radiosensitivity of tomato (Lycopersicon esculentum) and okra (Abelmoschus esculentus). Int J Agric Res 2(7):620–625
O’Kane D, Gill V, Boyd P, Burdon R (1996) Chilling, oxidative stress and antioxidant responses in. Arabidopsis thaliana callus Planta 198(3):371–377
Oviedo-Silva CA, Elso-Freudenberg M, Aranda-Bustos M (2018) L-DOPA trends in different tissues at early stages of Vicia faba growth: effect of tyrosine treatment. Appl Sci 8(12)
Patil A, Suryavanshi P, Fulzele D (2018a) In vitro regeneration of gamma irradiated callus of Artemisia annua and evaluation of increase artemisinin content by HPLC analysis. J Anal Pharm Res 7(5):569–573
Patil AS, Suryavanshi P, Fulzele D (2018b) Evaluation of effect of gamma radiation on total phenolic content, flavonoid and antioxidant activity of in vitro callus culture of Artemisia annua. Nat Prod Chem Res 6(6)
Patil R, Aware C, Gaikwad S, Rajebhosale M, Bapat V, Yadav S, Jadhav J (2019) RP-HPLC analysis of anti-parkinson’s drug l-DOPA content in Mucuna species from Indian Subcontinent. Proc Natl Acad Sci India Sect B - Biol Sci 89(4):1413–1420
Patil RR, Gholave AR, Jadhav JP, Yadav SR, Bapat VA (2014) Mucuna sanjappae Aitawade et Yadav: a new species of Mucuna with promising yield of anti-Parkinson’s drug L-DOPA. Genet Resour Crop Evol 62(1):155–162
Peltzer D, Dreyer E, Polle A (2002) Differential temperature dependencies of antioxidative enzymes in two contrasting species: Fagus sylvatica and Coleus blumei. Plant Physiol Biochem 40(2):141–150
Pugalenthi M, Vadivel V, Siddhuraju P (2005) Alternative food/feed perspectives of an underutilized legume Mucuna pruriens var. utilis - A review. Plant Foods Hum Nutr 60(4):201–218
Raaman N, Mummoorthy C, Swaminathan A (2013) Micropropagation of Mucuna pruriens (L.) DC and determination of tyrosinase. Med Plants 5(4):179–186
Raghavendra S, Ramesh CK, Kumar V, Khan MHM (2011) Elicitors and precursor induced effect on L-Dopa production in suspension cultures of Mucuna pruriens L. Front Life Sci 5(3–4):127–133
Rai SN, Chaturvedi VK, Singh P, Singh BK, Singh MP (2020) Mucuna pruriens in Parkinson’s and in some other diseases: recent advancement and future prospective. 3 Biotech 10(12):1–11
Rakesh B, Bindu KH, Praveen N (2021) Variations in the L-DOPA content, phytochemical constituents and antioxidant activity of different germlines of Mucuna pruriens (L.) DC. Asian J Chem 33(8):1881–1890
Rakesh B, Praveen N (2022) Establishment of Mucuna pruriens (L.) DC. callus and optimization of cell suspension culture for the production of anti-Parkinson’ s drug: L-DOPA. J Appl Biol Biotechnol 10:125–135. https://doi.org/10.7324/JABB.2022.100516
Randhir R, Kwon YI, Shetty K (2009) Improved health-relevant functionality in dark germinated Mucuna pruriens sprouts by elicitation with peptide and phytochemical elicitors. Bioresour Technol 100(19):4507–4514
Randhir R, Shetty P, Shetty K (2002) L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process Biochem 37(11):1247–1256
Rane M, Suryawanshi S, Patil R, Aware C, Jadhav R, Gaikwad S, Singh P, Yadav S, Bapat V, Gurav R, Jadhav J (2019) Exploring the proximate composition, antioxidant, anti-Parkinson’s and anti-inflammatory potential of two neglected and underutilized Mucuna species from India. South Afr J Bot 124:304–310
Saranya G, Jiby MV, Jayakumar KS, Padmesh Pillai P, Jayabaskaran C (2020) L-DOPA synthesis in Mucuna pruriens (L.) DC. is regulated by polyphenol oxidase and not CYP 450/tyrosine hydroxylase: An analysis of metabolic pathway using biochemical and molecular markers. Phytochemistry. 178(August 2019):112467
Sathish S, Vasudevan V, Karthik S, Elayaraja D, Pavan G, Ajithan C, Manickavasagam M (2020) Elicitors induced l-Dopa accumulation in adventitious root cultures of Hybanthus enneaspermus (L.) F. Muell Vegetos 33(2):304–312
Sathiyanarayanan L, Arulmozhi S (2007) Mucuna pruriens Linn. - A comprehensive review. Pharmacogn Rev 1(1):157–162
Savaskan C, Toker MC (1991) The effects of various doses gamma irradiation on the seed germination and root tips chromosomes of rye (Secales cereals L). Turk J Bot 15(February):349–359
El Sherif F, Khattab S, Ibrahim AK, Ahmed SA (2013) Improved silymarin content in elicited multiple shoot cultures of Silybum marianum L. Physiol Mol Biol Plants 19(1):127–136
Singh SK, Yadav D, Lal RK, Gupta MM, Dhawan SS (2016) Inducing mutations through γ-irradiation in seeds of Mucuna pruriens for developing high L-DOPA-yielding genotypes. Int J Radiat Biol:1–8
Suryawanshi S, Kshirsagar P, Kamble P, Bapat V, Jadhav J (2022) Systematic enhancement of L-DOPA and secondary metabolites from Mucuna imbricata: Implication of precursors and elicitors in callus culture. South Afr J Bot 144:419–429
Tandon B, Anand U, Alex BK, Kaur P, Nandy S, Shekhawat MS, Sanyal R, Pandey DK, Koshy EP, Dey A (2021) Statistical optimization of in vitro callus induction of wild and cultivated varieties of Mucuna pruriens L. (DC.) using response surface methodology and assessment of L-Dopa biosynthesis. Ind Crops Prod 169(May).
Tresina PS, Sornalakshmi V, Mohan VR (2018) Impact of gamma irradiation on the nutritional and antinutritional qualities of Mucuna deeringiana (Bort) Merril: an underexploited food legume. Int J Recent Res Asp (April):1010–1015
Ullah MA, Tungmunnithum D, Garros L, Drouet S, Hano C, Abbasi BH (2019) Effect of ultraviolet-C radiation and melatonin stress on biosynthesis of antioxidant and antidiabetic metabolites produced in in vitro callus cultures of Lepidium sativum L. Int J Mol Sci 20(7):1–19
Uma Sundaram AVG (2013) Optimization of pH and sucrose in the callus culture for the micropropagation of Mucuna pruriens using response surface methodology. Int J Pharm Pharm Sci 5:420–426
Vibha JB, Choudhary K, Singh M, Rathore MS, Shekhawat NS (2009) An efficient somatic embryogenesis system for velvet bean [Mucuna pruriens (L.) DC.]: A source of anti-Parkinson’s drug. Plant Cell Tiss Org Cult 99(3):319–325
Vishwakarma KS, Mohammed SI, Chaudhari AR, Salunkhe NS, Maheshwari VL (2017) Micropropagation and Agrobacterium rhizogenes mediated transformation studies in Mucuna pruriens (L.) DC. Indian J Nat Prod Resour 8:172–178
Wachisunthon D, Marsud S, Poonsatha S, Jetawattana S, Sitthithaworn W (2021) Productivity of L-DOPA in in vitro shoots of Mucuna pruriens var. utilis enhanced by gamma radiation. J Appl Pharm Sci 11(1):84–88
Wichers HJ, Wijnsma R, Visser JF, Malingré TM, Huizing HJ (1985) Production of L-DOPA by cell suspension cultures of Mucuna pruriens. Plant Cell Tiss Org Cult 4(1):75–82
Wise R, Naylor A (1987) Chilling-enhanced photooxidation: The peroxidative destruction of lipids during chilling injury to photosynthesis and ultrastructure. Plant Physiol 83(2):272–277
Author Information
Department of Life Sciences, CHRIST (Deemed to Be University), Bangalore, India