The improvement of PCR amplification pattern by PCR enhancers and formulation novel PCR enhancer
Short Communications | Published: 31 December, 2024
First Page: 2545
Last Page: 2554
Views: 2391
Keywords: PCR enhancers, PCR additives, Enzyme stabilizing compounds, Novel PCR enhancer, Sugars and Sugar alcohols
Abstract
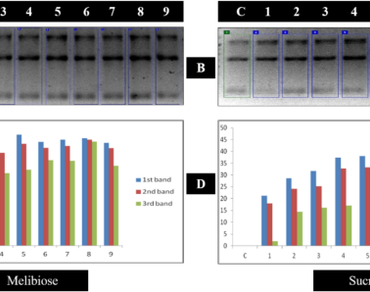
PCR protocols are always in need of improvement for scientific and industrial analysis. Numerous compounds have been shown to improve PCR amplification and function as PCR enhancers. Majority of PCR enhancement research used agarose gel electrophoresis to measure each enhancers concentration. The visual comparative observation is limited in the agarose gel electrophoresis, to facilitate the visual comparative analysis and to draw a conclusion, a computer-based gel analysis software was used. In the present study, eighteen enhancers were grouped into eight categories and tested in a range of concentrations. Among them, Glycerol and Trehalose were the only known kinds of sugars and sugar alcohols used as a PCR enhancers. We documented how eleven sugars and sugar alcohols enhance PCR amplification and reported that some of them were remarkably effective, often surpassing eighteen known PCR enhancers. We formulated novel PCR enhancers (NPEs) that greatly enhance the PCR process by lowering the melting temperature of DNA and thermostabilizing Taq DNA polymerase. A novel PCR enhancer is a beneficial and economically appealing for large-scale projects and routine applications that require reliable PCR results.
References
Adedokun G, Sidhu G, Alipanah M et al (2024) A handheld HIV detection platform using paper-based sample preparation and real-time isothermal amplification. Microsyst Nanoeng. https://doi.org/10.1038/s41378-024-00822-1
Aebischer A, Wernike K, Hoffmann B, Beer M (2014) Rapid genome detection of schmallenberg virus and bovine viral diarrhea virus by use of isothermal amplification methods and high-speed real-time reverse transcriptase PCR. J Clin Microbiol 52:1883–1892. https://doi.org/10.1128/jcm.00167-14
Aitchitt M, Ainsworth CC, Thangavelu M (1993) A rapid and efficient method for the extraction of total DNA from mature leaves of the date palm (Phoenix dactylifera L.). Plant Mol Biol Reptr 11:317–319
Bachmann B, Luke W, Hunsmann G (1990) Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucl Acids Res 18:1309
Back JF, Oakenfull D, Smith MB (1979) Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry 18:5191–5196
Chakrabarti R, Schutt CE (2001) The enhancement of PCR amplification by low molecular weight amides. Nucl Acids Res 29:2377
Chakrabarti R, Schutt CE (2002) Novel sulfoxides facilitate GC-rich template amplification. Biotechniques 32:866–874
Cheng S, Fockler C, Barnes WM, Higuchi R (1994) Effective amplification of long targets from cloned inserts and human genomic DNA. Proc Natl Acad Sci USA 91:5695–5699
Cleland W (1964) Dithiothreitol, a new protective reagent for SH groups. Biochemistry 3:480–482
Csako G (2006) Present and future of rapid and/or high-throughput methods for nucleic acid testing. Clin Chim Acta 363:6–31
Demeke T, Adams RP (1992) The effects of plant polysaccharides and buffer additives on PCR. Biotechniques 12:332–334
Doss RM, Marques MA, Foister S, Dervan PB (2004) Analysis of DNA minor groove recognition by the 3-methylthiophene/pyrrole pair. Chem Biodiv 1:886–889
Forootan A, Sjöback R, Björkman J, Sjögreen B, Linz L, Kubista M (2017) Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomole Detect Quantif 12:1–6. https://doi.org/10.1016/j.bdq.2017.04.001
Gao J, Huang S, Jiang J, Miao Q, Zheng R, Kang Y, Tang W, Zuo H, He J, Xie J (2024) Dual-CRISPR/Cas12a-assisted RT-RAA visualization system for rapid on-site detection of nervous necrosis virus (NNV). Anal Chim Acta 1335:343469. https://doi.org/10.1016/j.aca.2024.343469
Gibb K, Padovan A (1994) A DNA extraction method that allows reliable PCR amplification of MLO DNA from “difficult” plant host species. Genome Res 4:56–58
Gunasegar S, Neela VK (2021) Evaluation of diagnostic accuracy of loop-mediated isothermal amplification method (LAMP) compared with polymerase chain reaction (PCR) for Leptospira spp in clinical samples: a systematic review and meta-analysis. Diagn Microbiol Infect Dis 100:115369. https://doi.org/10.1016/j.diagmicrobio.2021.115369
Henke W, Herdel K, Jung K, Schnorr D, Loening SA (1997) Betaine improves the PCR amplification of GC-rich DNA sequences. Nucl Acids Res 25:3957–3958
Jensen MA, Fukushima M, Davis RW (2010) DMSO and betaine greatly improve amplification of GC rich constructs in De Novo synthesis. PLoS One 5:e11024
Kumar N, Khan A, Boora S, Chadha N, Khan N, Raina P, Gupta R, Singh R, Kaushik S (2024) Diagnosis of tuberculous lymphadenitis by molecular and immunological tools. Med Microecol 22:100116. https://doi.org/10.1016/j.medmic.2024.100116
Lestarini IA, Suryani D, Sabrina Y (2019) An efficient polymerase chain reaction (PCR) enhancer for highly guanine-cytosine (GC)-rich DNA sequences. Bali Med J 8:415
Lou XJ, Panaro NJ, Wilding P, Fortina P, Kricka LJ (2004) Increased amplification efficiency of microchip-based PCR by dynamic surface passivation. Biotechniques 36:248–252
Mandaliya VB, Pandya RV, Thaker VS (2010a) Single nucleotide polymorphism (SNP): a trend in genetics and genome analyses of plants. Gener Appli Plant Physiol 36:159–166
Mandaliya VB, Pandya RV, Thaker VS (2010b) Comparison of Cotton DNA extraction method for high yield and quality from various cotton tissue. J Cotton Res Dev 24:9–12
Mandaliya VB, Pandya RV, Thaker VS (2010c) Genetic diversity analysis of cotton (Gossypium) hybrids. J Cotton Res Dev 24:127–132
Mandaliya VB, Pandya RV, Thaker VS (2011) CSNP: a tool for harnessing the genetic potential of Cotton. Cotton Research J 2:1–14
Murray MG, Thomson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 19:4321–4325
Nagai M, Yoshida A, Sato N (1998) Additive effects of bovine serum albumin, dithiothreitol, and glycerol on PCR. Biochem Mol Biol Int 44:157–163
Nordstrom LJ, Clark CA, Andersen B, Champlin SM, Schwinefus JJ (2006) Effect of ethylene glycol, urea, and N-methylated glycines on DNA thermal stability: the role of DNA base pair composition and hydration. Biochemistry 45:9604–9614
Ouenzar B, Hartmann C, Rode A, Benslimane A (1998) Date palm DNA mini-preparation without liquid nitrogen. Plant Mol Biol Report 16:263–269
Petralia S, Nocito G, Conoci S, Sortino S (2020) Enhancement of PCR Reaction Efficiency by Gold-Nanoparticles Immobilized at Microreactor Surface. In: Di Francia G, Di Natale C, Alfano B, De Vito S, Esposito E, Fattoruso G, Formisano F, Massera E, Miglietta ML, Polichetti T (eds) Lecture notes in electrical engineering. Springer International Publishing, Cham, pp 183–187
Qiu Y, Zhang M, Yu Y, Wang A, Gao X (2013) The construction of pMD18-HT-Soybean as a calibrator plasmid and nested PCR assay for herbicide-tolerant soybeans. Eur Food Res Technol 238:375–386. https://doi.org/10.1007/s00217-013-2079-6
Ralser M, Querfurth R, Warnatz H, Lehrach H, Yaspo M, Krobitsch S (2006) An efficient and economic enhancer mix for PCR. Biochem Biophys Res Commun 347:747–751
Rees WA, Yager TD, Korte J, von Hippel PH (1993) Betaine can eliminate the base pair composition dependence of DNA melting. Biochemistry 32:137–144
Roux KH (1995) Optimization and troubleshooting in PCR. Genome Res 4:S185–S194
Sarkar G, Kapeiner S, Sommer SS (1990) Formamide can dramatically improve the specificity of PCR. Nucl Acids Res 18:7465
Schwartz AM, Fasman GD (1979) Thermal denaturation of chromatin and lysine copolymer-DNA complexes. Effects of ethylene glycol. Biopolymers 18:1045–1063
Shirasaki N, Matsushita T, Matsui Y, Koriki S (2020) Suitability of pepper mild mottle virus as a human enteric virus surrogate for assessing the efficacy of thermal or free-chlorine disinfection processes by using infectivity assays and enhanced viability PCR. Water Res 186:116409. https://doi.org/10.1016/j.watres.2020.116409
Simonovic A, Trifunovic M, Raspor M, Cingel A, Bogdanovic M, Dragicevic M, Subotic A (2012) Dimethyl sulfoxide improves sensitivity and specificity of RT-PCR and qRT-PCR amplification of low-expressed transgenes. Arch Biol Sci Belgrade 64:865–876
Spiess AN, Ivell R (2002) A highly efficient method for long-chain cDNA synthesis using trehalose and betaine. Anal Biochem 301:168–174
Spiess A, Mueller N, Ivell R (2004) Trehalose is a potent PCR enhancer: lowering of DNA melting temperature and thermal stabilization of Taq polymerase by the disaccharide trehalose. Clin Chem 50:1256–1259
Wanzhe Y, Jianuan L, Peng L, Jiguo S, Ligong C, Juxiang L (2015) Development of a nano-particle-assisted PCR assay for detection of duck tembusu virus. Lett Appl Microbiol 62:63–67. https://doi.org/10.1111/lam.12509
Xie B, Chen J, Wang Z, Yin Q, Dai Z (2024) Sweet enhancers of polymerase chain reaction. PLoS One 19:e0311939. https://doi.org/10.1371/journal.pone.0311939
Zafeiriadou A, Nano K, Thomaidis NS, Markou A (2024) Evaluation of PCR-enhancing approaches to reduce inhibition in wastewater samples and enhance viral load measurements. Sci Total Environ 955:176768. https://doi.org/10.1016/j.scitotenv.2024.176768
Zhang L, Liang Y, Meng L, Lu X, Liu Y (2007) Preparation and PCR-amplification properties of a novel amphiphilic poly(N-vinylpyrrolidone) (PVP) copolymer. Chem Biodiv 4:163–174
Author Information
Department of Microbiology, Faculty of Science, Marwadi University, Rajkot, India