Seasonal and solvent extraction influence on phenolic contents, antioxidant activity and antidiabetic capacity of three selected herbs from Chenopodiaceae family
Research Articles | Published: 03 January, 2024
First Page: 542
Last Page: 551
Views: 3041
Keywords: n A. alopecuroidesn , n C. Imbricatumn , n C. Vermiculatumn , Phenolic compound, Antioxidant, Antidiabetic
Abstract
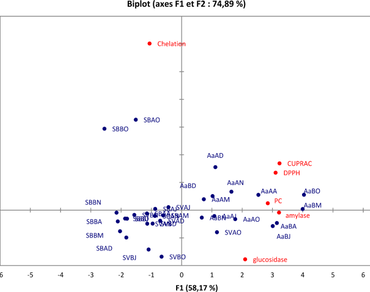
The aim of this research was to study seasonal influence on antioxidant activity, antidiabetic activity and phenolic content of three endemic plants belonging to Chenopodiaceae family: Caroxylon imbricatum (Forssk.) Akhani & Roalson, Caroxylon vermiculatum (L.) Akhani & Roalson and Agatophora alopecuroides (Delile) Bunge. The total phenolic content ranged from 15.98 to 0.61 mg gallic acid equivalents/g of dry weight where spring was found to be the best harvest season. The antioxidant activity of the butanolic fraction of A. alopecuroides (collected in October and May) measured by the DPPH and CUPRAC assays displayed the highest antioxidant activity (EC50 = 0.032 mg/mL and 0.016 mg/mL respectively).The ethyl acetate fraction of C. imbricatum (collected in October) displayed the highest iron chelating activity (EC50 = 0.141 mg/mL). All extracts inhibit α-amylase and α-glucosidase activities with different levels of inhibition more or less significant (3.39–91.01%). In order to have good inhibition of α-amylase and α-glucosidase tests, the best harvest season of A. alopecuroides was in May and in October for C. vermiculatum. For C. imbricatum the adequate harvest season for α-amylase test was in May and June for α-glucosidase test. This finding opens door to better use these natural plants in pharmacological products.
References
Al-Nablsi S, El-Keblawy A, Ali MA, Mosa KA, Alshaimaa M, Hamoda A, Shanableh AM, Almehdi, Sameh SM, Soliman (2022) Phenolic contents and antioxidant activity of Citrullus Colocynthis fruits, growing in the Hot Arid Desert of the UAE, Influenced by the Fruit Parts, Accessions, and Seasons of Fruit Collection. Antioxidants 11(4). https://doi.org/10.3390/antiox11040656
Al-Tohamy R, Samir Ali S, Saad-Allah K, Fareed M, Ali A, El-Badry A, Ahmed El-Zawawy N, Jian W, Jianzhong S, Guang-Hua M, Fatemeh RP (2018) Phytochemical analysis and assessment of antioxidant and antimicrobial activities of some medicinal plant species from Egyptian flora. J Appl Biomed 16:289–300
Amin E, Abdel-Bakky MS, Darwish MA, Mohammed HA, Chigurupati S, Qureshi KA, Hassan MHA (2022) The Glycemic Control Potential of some Amaranthaceae Plants, with Particular reference to in vivo antidiabetic potential of Agathophora alopecuroides. Molecules 27:973. https://doi.org/10.3390/molecules27030973
Ani VMC, Varadaraj K, Akhilender N (2006) Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum Nigrum L). Eur Food Res Technol 224:109–115
Athmouni K, Belghith T, Bellassouad K, Feki AE, Ayadi H (2015) Effect of extraction solvents on the biomolecules and antioxidant properties of scorzonera undulata (asteraceae), application of factorial design optimization phenolic extraction. Acta Scientiarum Polonorum Technologia Alimentaria 14(4):313–320. https://doi.org/10.17306/J.AFS.2015.4.32
Barrett AH, Farhadi NF, Smith TJ (2018) Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins, A review of efficacy and mechanisms. LWT Academic Press https://doi.org/10.1016/j.lwt.2017.09.002
Belhaoues S, Amri S, Bensouilah M (2020) Major phenolic compounds, antioxidant and antibacterial activities of Anthemis praecox Link aerial parts. South Afr J Bot 131:200–205. https://doi.org/10.1016/j.sajb.2020.02.018
Benhammou N, Ghambaza N, Benabdelkader S, Atik-Bekkara F, Kadifkova P (2013) Phytochemicals and antioxidant properties of extracts from the root and stems of Anabasis articulata. Int Food Res J 20(5):2057–2063
Biplab G, Sananda D, Tanaya D, Mrinmoy S, Jhimli B, Sandeep KD (2018) Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, Infection, cancer progression and other pathophysiological consequences, an update on glucose toxicity. Biomed Pharmacother 107:306–328. https://doi.org/10.1016/j.biopha.2018.07.157
Chen J, Yang J, Ma L, Li J, Shahzad N, Kim CK (2020) Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci Rep 10(1). https://doi.org/10.1038/s41598-020-59451-z
Ćorković I, Gašo-Sokač D, Pichler A, Šimunović J, Kopjar M (2022) Dietary polyphenols as natural inhibitors of α-Amylase and α-Glucosidase. Life (Basel Switzerland) 12(11):1692. https://doi.org/10.3390/life12111692
Djeridane A, Hamdi A, Bensania W, Cheifa K, Lakhdari I, Yousfi M (2015) The in vitro evaluation of antioxidative activity, α-glucosidase and α-amylase enzyme inhibitory of natural phenolic extracts. Diabetes and Metabolic Syndrome, Clinical Research and Reviews 9(4):324–331. https://doi.org/10.1016/j.dsx.2013.10.007
Dygert S, Li LH, Florida D, Thoma JA (1965) Determination of reducing sugar with improved precision. Anal Biochem 13(3):367–374. https://doi.org/10.1016/0003-2697(65)90327-1
Fereidoon S, JuDong Y (2018) Bioactivities of phenolics by focusing on suppression of chronic Diseases, a review. Int J Mol Sci 19(1573):1–16. https://doi.org/10.3390/ijms19061573
Feriha B, Brahim A, Reguia M, Amar D, Mohamed Y (2019) High antioxidant capacities and anti-inflammatory effects of Hammada Elegans Botsch. Extracts, an in vitro Assessment. Curr Enzym Inhib 15(1):55–68. https://doi.org/10.2174/1573408015666190225151916
Gammoh S, Alu’datt MH, Alhamad MN, Rababah T, Ereifej K, Almajwal A, Hussein NM (2017) Characterization of phenolic compounds extracted from wheat protein fractions using high-performance liquid chromatography/liquid chromatography mass spectrometry in relation to anti-allergenic, anti-oxidant, anti-hypertension, and anti-diabetic properties. Int J Food Prop 20(10):2383–2395. https://doi.org/10.1080/10942912.2016.1238832
de Gomes J Márcio Vinícius Cahino Terto, Sócrates Golzio do Santos, Marcelo Sobral Da Silva, and Josean Fechine Tavares. 2021. Seasonal variations of Polyphenols Content, Sun Protection Factor and antioxidant activity of two Lamiaceae Species. Pharmaceutics 13 (1): 1–16. https://doi.org/10.3390/pharmaceutics13010110
Hachani S, Aouissi H, Hamini F, and Mohamed Yousfi (2023) Algeria ” 臺灣農業化學與食品科學 61(2):103–110. https://doi.org/10.6578/TJACFS.202306_61(2).0005. “Antioxidant Activities of Butanolic Extracts of Phoenix Dactylifera Fruit Cultivars from Ain Salah
Hachani S, Hamia C, Boukhalkhal S, Silva AMS, Djeridane A, Yousfi M (2018) Morphological, physico-chemical characteristics and effects of extraction solvents on UHPLC-DAD-ESI-MSn profiling of phenolic contents and antioxidant activities of five date cultivars (Phoenix dactylifera L.) growing in Algeria. NFS J 13:10–22. https://doi.org/10.1016/j.nfs.2018.10.001
Hao L, Yan M, Jianglin Z, Jihua W, Ligang Z, Mingan W, Daoquan W, Jianguo H, Zhu Y, Fuyu Y (2010) Flavonoids from Halostachys Caspica and their antimicrobial and antioxidant activities. Molecules 15:7933–7945
Higginson R, Burrows P, Jones B (2019) Continuing Professional Development, Diabetes and associated diabetic emergencies. J Paramedic Pract 11(6):1–5. https://doi.org/10.12968/jpar.2019.11.6.cpd1
Jabri-Karoui I, Bettaieb I, Msaada K, Hammami M, Marzouk B (2012) Research on the phenolic compounds and antioxidant activities of Tunisian Thymus capitatus. J Funct Foods 4:661–669
Jemmali M, Kabana R (1972) Dosage rapide oxygraphique Du glucose en présence de glucose-oxydase dans les milieux complexes. Ann De Biol Animale Biochimie Biophysique 12(2):329–334. https://doi.org/10.1051/rnd,19720211
Kabubii ZN, Mbaria JM, Mathiu MP, Wanjohi JM, and Evans N. Nyaboga (2023) Evaluation of Seasonal Variation, Effect of extraction solvent on phytochemicals and antioxidant activity on Rosmarinus Officinalis grown in different agro-ecological zones of Kiambu County, Kenya. CABI Agric Bioscience 4(1):1–10. https://doi.org/10.1186/s43170-023-00141-x
Kamesh V, Hui-Fang C, Chin-Kun W (2019) Popular functional foods and herbs for the management of type-2-diabetes mellitus, a comprehensive review with special reference to clinical trials and its proposed mechanism. J Funct Foods 57:425–438. https://doi.org/10.1016/j.jff.2019.04.039
Khacheba I, Djeridane A, Kameli A, Yousfi M (2014) The Inhibitory Effect of some Algerian plants phenolics extracts on the α - glucosidase and α - amylase activities and their antioxidant activities. Curr Enzym Inhib 10(1):59–68. https://doi.org/10.2174/15734080113099990001
Liu Z, Yang Y, Dong W, Liu Q, Wang R, Pang J, Liu Y (2019) Investigation on the enzymatic profile of mulberry alkaloids by enzymatic study and molecular docking. Molecules 24(9) https://doi.org/10.3390/molecules24091776
Maire René (1949) (1878–1952). Flore de l’Afrique Du Nord (Maroc, Algérie, Tunisie, Tripolitaine, Cyrénaïque et Sahara). Edited by P. Lechevalier. Paris. paris, France. https://bibliotheques.mnhn.fr/medias/detailstatic.aspx?INSTANCE=exploitation&RSC_BASE=HORIZON&RSC_DOCID=35006&RSC_BASE=HORIZON&RSC_DOCID=35006
Martinez-Gonzalez AI, Díaz-Sánchez G, de la Rosa LA, Bustos-Jaimes I, Alvarez-Parrilla E (2019) Inhibition of α-amylase by flavonoids, structure activity relationship (SAR). Spectrochimica Acta - Part A Molecular and Biomolecular Spectroscopy 206:437–447. https://doi.org/10.1016/j.saa.2018.08.057
Mohammed HA, Al-Omar MS, Mohammed SAA, Alhowail AH, Eldeeb HM, Sajid MSM, Abd-Elmoniem EM, Alghulayqeh OA, Kandil YI, Khan RA (2021) Phytochemical analysis, pharmacological and safety evaluations of Halophytic Plant, Salsola Cyclophylla. Molecules 26:2384. https://doi.org/10.3390/molecules26082384
Moheylden AO, Ghada IM, Shaimaa SS (2020) Correlation between total phenols content, antioxidant power and cytotoxicity. Biointerface Res Appl Chem 11(3):10640–10653
Munteanu IG, Apetrei C (2021) Analytical methods used in determining antioxidant activity, a review. Int J Mol Sci 22:3380. https://doi.org/10.3390/ijms22073380
Oksana G, Chin-Kun W (2023) The hypoglycemic potential of phenolics from functional foods and their mechanisms. Food Sci Hum Wellness 12:986–10071
Othman A, Sayed AM, Amen Y, and Kuniyoshi Shimizu (2022) Possible neuroprotective effects of Amide alkaloids from Bassia Indica and Agathophora alopecuroides: in Vitro and in Silico investigations. RSC Adv 12(29):18746–18758. https://doi.org/10.1039/D2RA02275C
Ozenda P (1983) Flore Du Sahara. Edited by Centre national de la recherche scientifique. paris. 2nd ed
Quézel P, Santa Sébastien, Schotter O (1962) and Louis Emberger. “Nouvelle Flore de l’Algérie et Des Régions Désertiques Méridionales.”
Renata N, Katarzyna S, Urszula GD, Jolanta R, Lukasz K (2016) Antioxidative and cytotoxic potential of some Chenopodium L. species growing in Poland. Saudi J Biol Sci 23:15–23
Ribeiro DA, Camilo CJ, de Fátima Alves Nonato C, Rodrigues FFG, Menezes IRA, Ribeiro-Filho J, da Costa JGM (2020) Influence of seasonal variation on phenolic content and in vitro antioxidant activity of Secondatia floribunda A. DC. (Apocynaceae). Food Chemistry 315. https://doi.org/10.1016/j.foodchem.2020.126277
Serrano-Martínez A, Francisco dA, Maria IF, Carmen LA, Santiago LM, Estrella ND (2014) Effect of plant age and saline water on antioxidant and peroxidase activity in Sweet Pepper Fruit. J Agric Sci 6(12):139–151
Shakeri A, Nourallah H, Jafar V, Ali G, Fatemeh ZT (2012) Phytochemical screening, antimicrobial and antioxidant activities of Anabasis aphylla L. extracts. Kragujevac J Sci 34:71–78
Shehab NG, Abu-Gharbieh E, Medicine (2014) https://doi.org/10.1155/2014/695291
Singleton V, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:14–144
Stevanović ZD, Stanković MS, Stanković J, Janaćković P, Stanković M (2019) Use of halophytes as medicinal plants, phytochemical diversity and biological activity. In Halophytes and climate change, adaptive mechanisms and potential uses (pp. 343–358). https://doi.org/10.1079/9781786394330.0343
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ (2022) IDF Diabetes Atlas, Global, regional and country-level Diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109–119. https://doi.org/10.1016/j.diabres.2021.109119
Tahar M, Salim B, Mohamed T, Mohamed T (2019) The total phenolic compounds and antioxidant activity of Atriplex Nummularia leaves’ Extract. Int J Pharm Res Allied Sci 8(2):168–179
Tatipamula VB, Kukavica B (2021) Phenolic compounds as antidiabetic, anti-inflammatory, and anticancer agents and improvement of their bioavailability by liposomes. Cell Biochem Funct 39(8):926–944. https://doi.org/10.1002/cbf.3667
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-Induced Cancer. Chemico-Biol Interact 160(1):1–40
Xu QN, Zhu D, Wang GH, Lin T, Sun CL, Ding R, Tian WJ, Chen HF (2021) Phenolic glycosides and flavonoids with antioxidant and anticancer activities from Desmodium Caudatum. Nat Prod Res 35(22):4534–4541. https://doi.org/10.1080/14786419.2020.1739044
Yan L, Christian Z (2022) Seasonal variations of natural products in European herbs. Phytochem Rev 21:1549–1575. https://doi.org/10.1007/s11101-021-09797-7
Author Information
Departement de biologie, Université Kasdi Merbah, Ouargla, Algérie