Rice (Oryza sativa L.) landraces collected from Tamil Nadu, India shows enhanced level of nutritional composition and reduced in-vitro enzymatic digestibility
Research Articles | Published: 03 July, 2023
First Page: 1142
Last Page: 1152
Views: 3055
Keywords: Amylopectin chain length, Grain quality, In vitro starch digestibility, Principal component analysis, Rice landraces, Starch granule size
Abstract
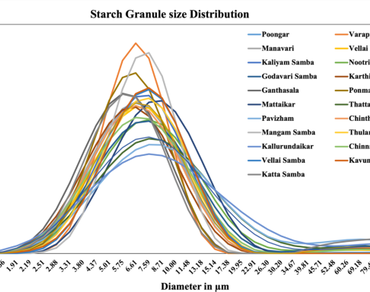
Traditional rice varieties have been neglected for human consumption and genetic improvement research. In the previous two decades, rice breeding initiatives have mostly concentrated on yield and yield contributing traits. In this study, 21 rice landraces were collected from various locations in Tamil Nadu, India, and their grain composition and enzymatic digestibility were evaluated. The rice landraces investigated showed significant differences in in vitro starch digestibility in meal and pure starch. Significant differences were also found in total dietary fiber (2.8–8.7), protein (6.2–12.3), fat (0.6–3.2%), and total phenol (1.3–6.9 mg GAE g-1). Katta Samba had the lowest hydrolysis index (HI) in both pure starch and meal samples. Except for Katta samba and Pavizham, B1 chains contributed more than 50% of total amylopectin in all landraces. The proportion of small (5 μm) starch granules ranged from 19.6 to 42.0%, while the proportion of medium (5–15 microns) size granules was between 52.6 and 79.8%. The percentage of large granules above 15 microns varied substantially from 0 to 21.0%. The principal component analysis showed that the first seven principal components explained 86.3% of the total variation. This splits the landraces into three subgroups with three outliers. The rice landraces that were found to have good grain qualities could be directly used for consumption or used in pre-breeding to develop rice varieties suitable for different end users.
References
Acquaah G (2012) Principles of Plant Genetics and breeding. Wiley-Blackwell Publication. http://onlinelibrary.wiley.com/book/10.1002/9781118313718 ISBN: 978-1-4051-3646-4
Adom KK, Sorrells ME, Liu RH (2003) Phytochemical profiles and antioxidant activity of wheat varieties. J Agric Food Chem 51:7825–7834. https://doi.org/10.1021/jf030404l
Agasimani S (2011) Isolation and characterization of mutants with high resistance starch (RS) content in rice (Oryza sativa L.), TNAU PhD Thesis
Ahuja G, Jaiswal S, Hucl P, Chibbar RN (2014) Differences in starch granule composition and structure influence in vitro enzymatic hydrolysis of grain meal and extracted starch in two classes of canadian wheat (Triticum aestivum L). Cereal Chem 91:233–239. https://doi.org/10.1094/CCHEM-07-13-0139-R
Asare EK, Jaiswal S, Maley J, Båga M, Sammynaiken R, Rossnagel BG, Chibbar RN (2011) Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J Agric Food Chem 59:4743–4754. https://doi.org/10.1021/jf200054e
Behall KM, Scholfield DJ (2005) Food amylose content affects postprandial glucose and insulin responses. Cereal Chem 82:654–659. https://doi.org/10.1094/CC-82-0654
Benmoussa M, Moldenhauer KA, Hamaker BR (2007) Rice amylopectin fine structure variability affects starch digestion properties. J Agric Food Chem 55:1475–1479. https://doi.org/10.1021/jf062349x
Bird AR, Flory C, Davies DA, Usher S, Topping DL (2004) A novel barley cultivar (Himalaya 292) with a specific gene mutation in starch synthase IIa raises large bowel starch and short-chain fatty acids in rats. J Nutr 134:831–835. https://doi.org/10.1093/jn/134.4.831
Calingacion M, Laborte A, Nelson A, Resurreccion A, Concepcion JC, Daygon VD, …, Fitzgerald M (2014) Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS ONE 9(1):e85106. https://doi.org/10.1371/journal.pone.0085106.g001
Chattopadhyay PK (2005) Postharvest technology for rice in India: a changing scenario. In: Rice is life: scientific perspectives for the 21st century (proceedings of the world rice research conference). 294–296
Chávez-Murillo CE, Méndez‐Montealvo G, Wang YJ, Bello‐Pérez LA (2012) Starch of diverse mexican rice cultivars: physicochemical, structural, and nutritional features. Starch‐Stärke 64:745–756. https://doi.org/10.1002/star.201200016
Deepa G, Singh V, Naidu KA (2010) A comparative study on starch digestibility, glycemic index and resistant starch of pigmented (‘Njavara’and ‘Jyothi’) and a non-pigmented (‘IR 64’) rice varieties. J Food Sci Technol 47:644–649. https://doi.org/10.1007/s13197-010-0106-1
Fu YB, Wangsomnuk PP, Ruttawat B (2014) Thai elite cassava genetic diversity was fortuitously conserved through farming with different sets of varieties. Conserv Genet 15:1463–1478. https://doi.org/10.1007/s10592-014-0631-y
Gani A, Wani SM, Masoodi FA, Salim R (2013) Characterization of rice starches extracted from indian cultivars. Food Sci Technol Int 19:143–152. https://doi.org/10.1177/1082013212442189
Goñi I, García-Alonso A, Saura-Calixto F (1997) A starch hydrolysis procedure to estimate glycemic index. Nutr Res 17:427–437. https://doi.org/10.1016/S0271-5317(97)00010-9
Gurunathan S, Ramadoss BR, Nayak C, Kalagatur NK, Bapu JR, Mohan C, Alqarawi AA, Hashem A, Abd_Allah EF (2019) Single nucleotide polymorphisms (SNPs) in starch biosynthetic genes associated with increased resistant starch concentration in rice mutant. Front Genet 10:946. https://doi.org/10.3389/fgene.2019.00946
Hanashiro I, Abe JI, Hizukuri S (1996) A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr Res 283:151–159. https://doi.org/10.1016/0008-6215(95)00408-4
Hill JH, Becker C, Tigerstedt PMA (1998) Quantitative and ecological aspects of plant breeding. Chapman and Hall, London, p 275
Hu P, Zhao H, Duan Z, Linlin Z, Wu D (2004) Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J Cereal Sci 40:231–237. https://doi.org/10.1016/j.jcs.2004.06.001
Hudson EA, Dinh PA, Kokubun T, Simmonds MSJ, Gescher A (2000) Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomark Prev 9:1163–1170
Jaiswal S, Båga M, Ahuja G, Rossnagel BG, Chibbar RN (2014) Development of barley (Hordeum vulgare L.) lines with altered starch granule size distribution. J Agric Food Chem 62:2289–2296. https://doi.org/10.1021/jf405424x
Juliano BO, Bautista GM, Lugay JC, Reyes ACJ (1964) Studies on the physicochemical properties of rice. Agric Food Chem 12:131–138. https://doi.org/10.1021/jf60132a010
Kesarwani A, Chiang PY, Chen SS (2014) Distribution of phenolic compounds and antioxidative activities of rice kernel and their relationships with agronomic practice. Sci World J 2014:1–6. https://doi.org/10.1155/2014/620171
Khush GS (2005) What it will take to feed 5.0 billion rice consumers in 2030? Plant Mol Biol 59:1–6. https://doi.org/10.1007/s11103-005-2159-5
Kim JK, Lee SY, Chu SM, Lim SH, Suh SC, Lee YT, Cho HS, Ha SH (2010) Variation and correlation analysis of flavonoids and carotenoids in korean pigmented rice (Oryza sativa L.) cultivars. J Agric Food Chem 58:12804–12809. https://doi.org/10.1021/jf103277g
Krygier K, Sosulski F, Hogge L (1982) Free, esterified, and insoluble-bound phenolic acids. 1. Extraction and purification procedure. J Agric Food Chem 30:330–334. https://doi.org/10.1021/jf00110a028
Lumdubwong N, Seib PA (2000) Rice starch isolation by alkaline protease digestion of wet-milled rice flour. J Cereal Sci 31:63–74. https://doi.org/10.1006/jcrs.1999.0279
Miller JB, Pang E, Bramall L (1992) Rice: a high or low glycemic index food? Am J Clin Nutr 34:1034–1036. https://doi.org/10.1093/ajcn/56.6.1034
Mohan V, Spiegelman D, Sudha V, Gayathri R, Hong B, Praseena K, Anjana RM, Wedick NM, Arumugam K, Malik V, Ramachandran S, Ramya bai M, Henry JK, Hu FB, Willett W, Krishnaswamy K (2014) Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight asian Indians: a randomized controlled trial. Diabetes Technol Ther 16:317–325. https://doi.org/10.1089/dia.2013.0259
Morrison WR, Milligan TP, Azudin MN (1984) A relationship between the amylose and lipid contents of starches from diploid cereals. J Cereal Sci 2:257–271. https://doi.org/10.1016/S0733-5210(84)80014-4
Nachimuthu V, Robin S, Sudhakar D, Raveendran M, Rajeswari S, Manonmani S (2014) Evaluation of rice genetic diversity and variability in a population panel by principal component analysis. Indian J Sci Technol 7:1555–1562
Nachimuthu V, Sudhakar D, Raveendran M, Rajeswari S, Manonmani S, Tannidi S, Aravindhan PB, Ponniah G, Gunasekaran K, Robin S (2017) Rice Diversity Panel evaluated for agro-morphological diversity by Multivariate Analysis. Int J Curr Microbiol App Sci 6:3887–3901. https://doi.org/10.20546/ijcmas.2017.611.455
Plastina P, Gabriele B, Fazio A (2018) Characterizing traditional rice varieties grown in temperate regions of Italy: free and bound phenolic and lipid compounds and in vitro antioxidant properties. Food Qual Saf 2:89–95. https://doi.org/10.1093/fqsafe/fyy005
Popkin BM (2006) Global nutrition dynamics: the world is shifting rapidly toward a diet linked with non-communicable diseases. Am J Clin Nutr 84:289–298. https://doi.org/10.1093/ajcn/84.2.289
Raja RB, Agasimani S, Jaiswal S, Thiruvengadam V, Sabariappan R, Chibbar RN, Ram SG (2017b) EcoTILLING by sequencing reveals polymorphisms in genes encoding starch synthases that are associated with low glycemic response in rice. BMC Plant Biol 17(1):1–13. https://doi.org/10.1186/s12870-016-0968-0
Raja RB, Anusheela V, Agasimani S, Jaiswal S, Thiruvengadam V, Chibbar RN, Ram SG (2017a) Validation and applicability of single Kernel-Based Cut Grain Dip Method for Amylose determination in Rice. Food Anal Methods 10:442–448. https://doi.org/10.1007/s12161-016-0607-2
Raji AA (2002) Assessment of genetic diversity and heterotic relationships in African improved and local cassava (Manihot esculenta Crantz) germplasm. PhD thesis, University of Ibadan, Ibadan, Nigeria
Ramadoss BR, Gangola MP, Agasimani S, Jaiswal S, Venkatesan T, Sundaram GR, Chibbar RN (2019) Starch granule size and amylopectin chain length influence starch in vitro enzymatic digestibility in selected rice mutants with similar amylose concentration. J Food Sci Technol 56(1):391–400. https://doi.org/10.1007/s13197-018-3500-8
Regina A, Bird A, Topping D, Bowden S, Freeman J, Barsby T, Kosar-Hashemi B, Li Z, Rahman S, Morell M (2006) High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc Natl Acad Sci USA 103:3546–3551. https://doi.org/10.1073/pnas.0510737103
Rao ASVC, Sareddy GR, Phanithi PB, Attipalli RR (2010) The antioxidant and antiproliferative activities of methanolic extracts from Njavara rice bran. Complement Alternat Med 10:4–9. https://doi.org/10.1186/1472-6882-10-4
Roy S, Marndi BC, Mawkhlieng B, Banerjee A, Yadav RM, Misra AK, Bansal KC (2016) Genetic diversity and structure in hill rice (Oryza sativa L.) landraces from the North-Eastern Himalayas of India. BMC Genet 17:107. https://doi.org/10.1186/s12863-016-0414-1
Samal R, Roy PR, Dash AK, Rao GJN, Bharathkumar S, Subudhi HN, Reddy JN (2016) Genetic diversity in the rice landraces (Oryza sativa L.) of coastal Sundarbans (India) and their adaptation to the local saline condition investigated both at molecular and physiological level. Acta Physiol Plant 38:56. https://doi.org/10.1007/s11738-015-2046-x
Sang Y, Seib PA (2006) Resistant starches from amylose mutants of corn by simultaneous heat-moisture treatment and phosphorylation. Carbohydr Polym 63:167–175. https://doi.org/10.1016/j.carbpol.2005.07.022
Valarmathi R, Raveendran M, Robin S, Senthil N (2015) Unraveling the nutritional and therapeutic properties of ‘Kavuni’a traditional rice variety of Tamil Nadu. J Plant Biochem Biot 24:305–315. https://doi.org/10.1007/s13562-014-0274-6
Vichapong J, Srijesdaruk M, Srijesdaruk V, Swatsitang P, Srijaranai S (2010) High performance liquid chromatographic analysis of phenolic compounds and their antioxidant activities in rice varieties. LWT Food Sci Technol 43:1325–1330. https://doi.org/10.1016/j.lwt.2010.05.007
Villegas R, Liu Y, Gao T (2007) Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged chinese women. Arch Intern Med 167:2310–2316. https://doi.org/10.1001/archinte.167.21.2310
Walter M, da Silva LP, Denardin CC (2005) Rice and resistant starch: different content depending on chosen methodology. J Food Compost Anal 18:279–285. https://doi.org/10.1016/j.jfca.2004.09.007
Wang L, Xie B, Shi J, Xue S, Deng Q, Wei Y, Tian B (2010) Physicochemical properties and structure of starches from chinese rice cultivars. Food Hydrocoll 24:208–216. https://doi.org/10.1016/j.foodhyd.2009.09.007
Zhu LJ, Liu QQ, Sang Y, Gu MH, Shi YC (2010) Underlying reasons for waxy rice flours having different pasting properties. Food Chem 120:94–100. https://doi.org/10.1016/j.foodchem.2009.09.076
Author Information
Department of Plant Sciences, University of Saskatchewan, Saskatoon, Saskatchewan, Canada