Potential inhibition of entomopathogenic nematodes and plant growth-promoting bacteria with exposure to selected herbicides and insecticides
Fabiyi Oluwatoyin Adenike, Adebisi Olusoji Olusegun, Falore Sunday Olubusuyi, Claudius-Cole Abiodun Olufunmilayo
Research Articles | Published: 21 August, 2023
First Page: 1503
Last Page: 1512
Views: 2865
Keywords: Bacteria, n Heterorhabdidtis sp., Paraquat, Imidacloprid, Pollution, EPN, Toxicity
Abstract
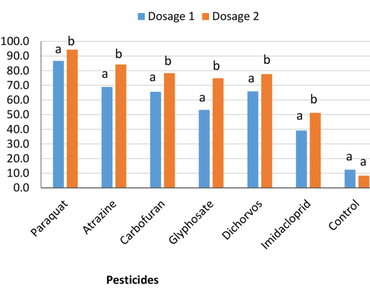
The inherent dangers of pesticide pollution due to non-judicious use by farmers in sub-Saharan Africa is a cause for concern in soil biodiversity conservation. To this effect, this study investigated the consequence of common pesticides used in Nigeria on the survival and population of entomopathogenic nematodes (EPNs) and plant growth promoting bacteria (PGPB) under laboratory conditions. The selected pesticides were insecticides (dichlorvos, imidacloprid and carbofuran (also a nematicide) and herbicides (atrazine, glyphosate and paraquat). Nine PGPB and a local isolate of EPN were evaluated. The effect of the selected insecticides and herbicides on EPN and PGPB was dependent on exposure time, pesticide type and their concentration. Percentage mortality of infective juveniles (Ijs) of EPN was significantly higher with exposure to the pesticides (p < 0.05) at 24 h of exposure in comparison with control. Mortality of EPN Ijs was above 85% in paraquat, atrazine, carbofuran, glyphosate and dichlorvos after 72 h of exposure to x2 dosage, while 70% was recorded in imidacloprid. Poor colony formation was noted among all PGPB isolates regardless of exposure time. The highest colony units/ml (130.33 units/ml) was recorded in carbofuran. Colony formation was significantly (p < 0.05) low in media tainted with dichlorvos (11.33 units/ml), while 257.33 units/ml was recorded in the non-treated plates. The effect of pesticides on PGPB was a factor of species and type of pesticide. Psedomonas plecoglossicida was the least affected after 72 h of exposure (124.9 colony units/ml) whereas Leclercia adecarboxylata was the most adversely affected (67.3 colony units/ml). Reduction in colony formation is a hindrance to the traditional soil conditioning role of PGPB, the undesirable effect of pesticides on EPN populations is equally a limiting factor in EPN-insecticide combination control methods. Environmental and health risks associated with pesticide use should be considered before embarking on pesticide application. The existing approach in agriculture, where pesticide use is primary should be discouraged to forestall huge ecological damage.
References
Abate BA, Wingfield MJ, Slippers B, Hurley BP (2017) Commercialisation of entomopathogenic nematodes: should import regulations be revised? Biocontrol Sci Tech 27(2):149–168
Ahemad, Khan (2010) Influence of selective herbicides on plant growth promoting traits of phosphate solubilising Enterobacter asburiae strain PS2. Res J Microbiol 5(9):849–857
Ahemad M, Khan MS (2011b) Ecotoxicological assessment of pesticides towards the plant growth promoting activities of Lentil (Lens esculentus)-specific Rhizobium sp. strain MRL3 ecotoxicology. 20:661–669. https://doi.org/10.1007/s10646-011-0606-4
Ahemad M, Khan MS (2011a) Toxicological assessment of selective pesticides towards plant growth promoting activities of phosphate solubilizing Pseudomonas aeruginosa Acta Microbiol. Immunol Hungarica 58(3). https://doi.org/10.1556/AMicr.58.2011.3.1
Bora S, Vardhan GSH, Deka N, Khataniar L, Gogoi D, Baruah A (2021) Paraquat exposure over generation affects lifespan and reproduction through mitochondrial disruption in C. elegans. Toxicology 447:152632
Burul F, Baric K, Lakić J, Milanović-Litre A (2022) Herbicides effects on symbiotic nitrogen-fixing bacteria. J Cent Eur Agric 23(1):89–102. https://doi.org/10.5513/JCEA01/23.1.3320
Chandra S, Chakraborty D (2023) Survivability and plant growth-promoting traits of Rhizobium aegyptiacum under the stress of fungicides and insecticides. Mycopath 21(1):1–5
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils 48:489–499
Chavan SN, Somasekhar N, Katti G (2018) Compatibility of entomopathogenic nematode Heterorhabditis indica (Nematoda: Heterorhabditidae) with agrochemicals used in the rice ecosystem. J Entomol Zool Stud 6:527–532
Chen M, Zhu B, Lin L, Yang L, Li Y, An Q (2014) Complete genome sequence of Kosakonia sacchari type strain SP1. Stand Genomic Sci 9(3):1311–1318. https://doi.org/10.4056/sigs.5779977
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Soil Ecol 36(23):84–189. https://doi.org/10.1016/j.apsoil.2007.02.005
Eisenhauer N, Klier M, Partsch S, Sabais ACW, Scherber C, Weisser WW, Scheu S (2009) No interactive effects of pesticides and plant diversity on soil microbial biomass and respiration. Appl Soil Ecol 42(1):31–36
Fabiyi OA, Olatunji GA (2021) Environmental sustainability: Bioactivity of Leucaena leucocephala Leaves and Pesticide Residue analysis in Tomato Fruits. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 69(4):473–480
Fabiyi OA (2023) The Perspective of Nanotechnology in Plant Protection. In Nanophytopathology. Avila-Quezada, G. D and Rai, M. (Eds). 23-38Pp. doi https://doi.org/10.1201/9781003344513-3
Fabiyi OA, Adebisi O, Falore S, Bello TT, Olatunji G (2023) Assessment of Actinomycetes and Psedomonas species on Meloidogyne incognita population and growth of carrot plants in disparate soils. Indian Phytopathol 76:593–604. https://doi.org/10.1007/s42360-023-00629-6
Fabiyi OA, Baker MT, Claudius-Cole AO, Alabi RO, Olatunji GA (2022) Application of cyclic Imides in the management of Root-Knot Nematode (Meloidogyne incognita) on Cabbage. Indian Phytopathol 75(2):457–466. https://doi.org/10.1007/s42360-022-00480-1
Garcia-del-Pino F, Morton A, Shapiro-Ilan D (2018) Chapter 12 - entomopathogenic nematodes as Biological Control Agents of Tomato Pests. Sustainable Manage Arthropod Pests Tomato 12:269–282. https://doi.org/10.1016/B978-0-12-802441-6.00012-7
Glick BR (2012) Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica. 1–15. https://doi.org/10.6064/2012/963401
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin H, Sand Patra JK (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microb Res 206:131–140. https://doi.org/10.1016/j.micres.2017.08.016
Hara AH, Kaya HK (1983) Toxicity of selected organophosphate and carbamate pesticides to infective juveniles of the entomogenous nematode Neoaplectana carpocapsae (Rhabditida: Steinernematidae). Env Entomol 12(2):496–501
Hardoim PR, Nazir R, Sessitsch A, Elhottová D, Korenblum E, Overbeek LS, Elsas JD (2013) The new species Enterobacter oryziphilus sp. nov. and Enterobacter oryzendophyticus sp. nov. are key inhabitants of the endosphere of rice. BMC Microbiol 13:164. https://doi.org/10.1186/1471-2180-13-164
Helmberger MS, Shields EJ, Wickings KG (2017) Ecology of below ground biological control: entomopathogenic nematode interactions with soil biota. Appl Soil Ecology 121:201–213. https://doi.org/10.1016/j.apsoil.2017.10.013
Jetiyanon K, Pinyupa P (2016) Lipopolysaccharide of Enterobacter asburiae strain RS83: a bacterial determinant for induction of early defensive enzymes in Lactuca Sativa against Soft Rot Disease. " Biol Control 67(3):301–307
Kang SM, Radhakrishnan R, You YH, Khan AL, Lee KE, Lee JD, Lee IJ (2015) Enterobacter asburiae’’KE17 association regulates physiological changes and mitigates the toxic effects of heavy metals in soybean. Plant Biol 17:1013–1022
Krishnayya PV, Grewal PS (2002) Effect of neemand selected fungicides on viability and virulence of the entomopathogenic nematode Steinernema feltiae. Biocontrol Sci Tech 12:259–266
Kumar A, Singh R, Yadav A, Giri DD, Singh PK, Pandey KD (2016) Isolation and characterization of bacterial endophytes of Curcuma longa (L.) 3. Biotechnology 6:60
Lacey LA, Georgis R (2012) Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J Nematology 44(2):218–225
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebrate Pathol 132:1–41
Laurentis VL, Bortoli SA, Polanczyk RA, Vacari AM, Veiga ACP, Bortoli CP, Volpe HXL (2014) Kluyvera ascorbata: a plant growth-promoting Bacteria (PGPB) to Manage Plutella xylostella (L., 1758) (Lepidoptera: Plutellidae). Inter J Res Agric Sci 1(5):340–343
Laznik Ž, Trdan S (2014) The influence of insecticides on the viability of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) under laboratory conditions. Pest managt sci 70(5):784–789
Meena RS, Kumar S, Datta R, Lal R, Vijayakumar V, Brtnicky M, Sharma MP, Yadav GS, Jhariya MK, Jangir CK, Pathan SI (2020) Impact of agrochemicals on soil microbiota and management: A review. Land, 9(2), p.34
Navarro PD, McMullen JG, Stock SP (2014) Effect of dinotefuran, indoxacarb, and imidacloprid on survival and fitness of two arizona-native entomopathogenic nematodes against Helicoverpa zea (Lepidoptera: Noctuidae). Nematropica. 64(1):44–73
Nie M, Nie H, He M, Lin Y, Wang L, Jin P, Zhang S (2016) Immobilization of biofilms of Pseudomonas aeruginosa NY3 and their application in the removal of hydrocarbons from highly concentrated oil-containing wastewater on the laboratory scale. J Environ Manag 173:34–40. https://doi.org/10.1016/j.jenvman.2016.02.045
Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J Microb Biotechnol 6(11):197. https://doi.org/10.1007/s11274-017-2364-9
Ottun A, Alabi O, Claudius-Cole AO (2021) Evaluation of native Entomopathogenic Nematode isolates for the management of selected insect pests in Nigeria. Egypt J Agronematology 20(2):167–176
Özdemir E, İnak E, Evlice E, Laznik Z (2020) Compatibility of entomopathogenic nematodes with pesticides registered in vegetable crops under laboratory conditions. J plt dis & prot 127:529–535
Patel LC (2021) Compatibility of entomopathogenic nematode, Steinernema sp. and egg parasitoid, Trichogramma japonicum with insecticides. J Entomol Res 45(suppl):862–865
Qu Q, Li Y, Zhang Z, Cui H, Zhao Q, Liu W, Lu T, Qian H (2021) Effects of S-metolachlor on wheat (Triticum aestivum L.) seedling root exudates and the rhizosphere microbiome. J Hazard Mater 411:125137
Ramakrishna W, Yadav R, Li K (2019) Plant growth promoting bacteria in agriculture: two sides of a coin. Appl Soil Ecol 138:10–18. https://doi.org/10.1016/j.apsoil.2019.02.019
Rovesti L, Deseö KK (1990) Compatibility of chemical pesticides with entomopathogenic nematodes, Steinernema carpocapsae Weiser and S. feltiae Filipjev (Nematoda: Steinernematidae). Nematologica 36:237–245
Rovesti L, Heinzpeter EW, Tagliente F, Deseö KV (1988) Compatibility of pesticides with the entomopathogenic nematode Heterorhabditis Bacteriophora Poinar (Nematoda: Heterorhabditidae). Nematology 34:462–476
Sanchis V and Bourguet. D (2008) Bacillus thuringiensis: applications in agriculture and insect resistance management. A review. Agron Sus Dev 28(1):11–20
Sándor Z (2006) Különböző herbicidek hatása a talajbanélő mikrobák mennyiségi előfordulásáraés aktivitására Agrártudományi Közlemények. 23:76–82
Sándor Z (2010) Examination the effects of different herbicides on the soil microorganisms of a calcareous chernozem. J Agricultural Sci Debrecen 38:121–126
Sato V, Sayuri, Renato F, Galdiano Júnior GR, Rodrigues, Eliana GM, Lemos, João Martins Pizauro Junior (2016) Kinetic characterization of a Novel Acid Ectophosphatase from Enterobacter asburiae. J Microbiol 54(2):106–113
Shapiro DI, Lewis EE (1999) Comparison of entomopathogenic nematode infectivity from infected hosts versus aqueous suspension. Environ Entomol 28(5):907–911
Storchous I, Stefkivska Yu (2019) Ammonium glufosinate and triazine herbicides have side effects on soil microorganisms and pathogens. ПОБІЧНИЙ ВПЛИВ 2579–10. https://doi.org/10.36495/2312-0614.2019.9-10.6-11
Sturhan D, Mracek Z (2000) Comparison of the Galleria baiting technique and a direct extraction method for recovering Steinernema (Nematoda: Rhabditida) infective-stage juveniles from soil. Folia Parasitol 47(4):315–318
Teng Z, Shao W, Zhang K, Huo Y, Zhu J, Li M (2019) Pbbisorption by Leclercia adecarboxylata: protective and immobilized mechanisms of extracellular polymeric substances. Chem Engin J 375(1). https://doi.org/10.1016/j.cej.2019.122113
Timmusk S, Paalme V, Pavlicek T (2011) “Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates,” PLoS ONE, vol. 6, no. 3, Article ID e17968, 2011
Vashisth S, Chandel YS, Sharma PK (2012) Entomopathogenic nematodes - a review. Agric Reviews 34(3):163–175. https://doi.org/10.5958/j.0976-0741.34.3.001
Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq BA (2016) Role of Plant Growth promoting Rhizobacteria in Agricultural Sustainability-A review. Molecules 21(5):E573. https://doi.org/10.3390/molecules21050573
Xie H, Wang X, Zhang L, Wang T, Zhang W, Jiang J, Chang PK, Chen ZY, Bhatnagar D, Zhang Q, Li P (2018) Monitoring metabolite production of aflatoxin biosynthesis by orbitrap fusion mass spectrometry and a D-optimal mixture design method. Anal Chem 90:14331–14338. https://doi.org/10.1021/acs.analchem.8b03703
Xu N, Qu Q, Zhang Z, Yuan W, Cui H, Shen Y, Lin W, Lu T, Qian H (2020) Effects of residual S-metolachlor in soil on the phyllosphere microbial communities of wheat (Triticum aestivum L). Sci Total Environ 748:141342
Yin H, Qiang J, Jia Y, Ye J, Peng H, Qin H, Zhang N, He B (2009) Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil-containing wastewater. Proc. Biochem. 44 (3): 302–308. https://doi.org/10.1016/j.procbio.2008.11.003
Zarraonaindia I, Weisenhorn MOS, West P, Hempton-Marcell K, Lax J, Bokulich S, Mills NA, Martin DA, Taghavi G, Van der Lelie S, Gilbert DJA (2015) The soil microbiome influences grape vine associated mecrobiota. Mbio 6:e02527–e02514
Zhou C, Ge N, Guo J, Zhu L, Ma Z, Cheng S, Wang J (2019) Enterobacter asburiae reduces Cadmium Toxicity in Maize plants by repressing Iron Uptake-Associated Pathways. J Agric Food Chem 67(36):10126–10136. https://doi.org/10.1021/acs.jafc.9b03293
Zimmerman RJ, Cranshaw WS (1990) Compatibility of three entomopathogenous nematodes (Rhabditida) in aqueous solutions of pesticides used in turf grass maintenance. J Econ Entomol 83:97–100
Author Information
Department of Crop Protection, Faculty of Agriculture, University of Ilorin, Ilorin, Nigeria