Physiological responses shown by the apical (green) and basal (brown) leaves of seven taxa of moss family Pottiaceae (Bryophyta): A comparative study from India
Research Articles | Published: 05 January, 2024
First Page: 1938
Last Page: 1947
Views: 3132
Keywords: Antioxidants, Apical green leaves, Basal brown leaves, Moss, Pottiaceae
Abstract
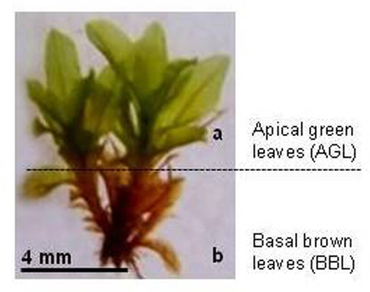
Mosses synthesize and preserve a diverse array of secondary metabolites which play a pivotal role in enhancing plant adaptability to environmental fluctuations. These compounds manage reactive oxygen species (ROS) and prevent the uncontrolled oxidation of essential biomolecules. Despite numerous studies highlighting the antibacterial, antifungal, anti-inflammatory, antipyretic, and insecticidal properties of mosses, the antioxidant capacity of mosses remained relatively understudied, with existing research findings dispersed. This investigation aims to evaluate the antioxidant potential and pigment composition of apical green and basal brown leaves of seven species belonging to one of the stress tolerant moss family i.e. Pottiaceae, collected from semi-arid regions of Rajasthan. Remarkable variations in enzymatic responses and chlorophyll concentration were noted among different taxa within the family. The apical green leaves of Hydrogonium consanguineum exhibited the highest superoxide dismutase (SOD) activity, whereas Didymodon vinealis and Hyophila involuta displayed significantly elevated levels of catalase (CAT) and peroxidase (POD), respectively. While previous studies have explored antioxidant activity in various plant parts of angiosperms, such as roots, mature leaves, young leaves, flowers, and fruits, no comparable research has been conducted on moss species within a single family, particularly evaluating apical green and basal brown leaves. Our findings suggest that in contrast to brown leaves, green leaves possess higher antioxidant activity. However, further research is imperative to comprehensively comprehend the antioxidant capacities and active metabolites of the examined moss species.
References
Ah-Peng C, Cardoso AW, Flores O, West A, Wilding N, Strasberg D, Hedderson TAJ (2017) The role of epiphytic bryophytes in interception, storage, and the regulated release of atmospheric moisture in a tropical montane cloud forest. J Hydrol 548:665–673
Arnon DI (1949) Copper enzyme polyphenoloxides in isolated chloroplast in Beta vulgaris. Plant Physiol 24:1–15
Asakawa Y, Ludwiczuk A, Nagashima F (2013) Phytochemical and biological studies of bryophytes. Phytochemistry 91:52–80
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: An overview. Photosynthetica 51:163–190
Aziz MN, Vohra JN (2008) Pottiaceae (Musci) of India. Bishen Singh Mahendra Pal Singh, Dehra Dun, India
Bakardjieva NT, Christova N, Christov K (1996) Effect of calcium and zinc ions on the sensitivity of peroxidise from mosses (Mnium sp.) and ferns (Polypodium vulgare) to high temperature. Can J Bot 74:1665–1670
Bansal P, Srivastava A (2017) Desiccation-related responses of antioxidative enzymes and photosynthetic pigments in Brachythecium procumbens (Mitt.) A. Jaeger. Acta Physiol Plant 39:154
Bansal P, Srivastava A (2020) An adaptive inducible ecological tolerance strategy of Semibarbulaorientalis (Web.) Wijk. & Marg. to desiccation stress. Plant Physiol Rep 25:460–471
Bansal P, Verma S, Srivastava A (2016) Biomonitoring of air pollution using antioxidative enzyme system in two genera of family Pottiaceae (Bryophyta). Environ Pollut 216:512–518
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an application to acrylamide gels. Anal Biochem 44:276–287
Buraphaka H, Puttha W, Putalun W (2022) Comparative evaluation of antioxidant and anti-inflammatory activity of active compounds identified in Ardisia elliptica extracts from different plant parts. Chem Biodivers 19(2):e202100796
Chobot V, Kubicová L, Nabbout S, Jahodár L, Hadacek F (2008) Evaluation of antioxidant activity of some common mosses. Z Naturforsch C 63:476–482
Chobot V, Kubicová L, Nabbout S, Jahodář L, Vytlačilová J (2006) Antioxidant and free radical scavenging activities of five moss species. Fitoterapia 77:598–600
Cianciullo P, Maresca V, Sorbo S, Basile A (2022) Antioxidant and antibacterial properties of extracts and bioactive compounds in Bryophytes. Appl Sci 12(1):160
Cruz de Carvalho R, Branquinho C, Marques da Silva J (2019) Desiccation rate affects chlorophyll and carotenoid content and the recovery of the aquatic moss Fontinalis antipyretica (Fontinalaceae). Hattoria 10:53–60
Dumanovic J, Nepovimova E, Natic M, Kuca K, Jacevic V (2021) The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front Plant Sci 11:1–13
Ertürk Ö, Sahin H, Ertürk EY, Hotaman HE, Koz B, Özdemir Ö (2015) The antimicrobial and antioxidant activities of extracts obtained from some moss species in Turkey. Herba Pol 61(4):52–65
Euler HV, Josephson K (1927) Uber katalase. I. Justus Liebigs Annalen Der Chemie 452:158–181
Frederick SE, Gruber PJ, Tolbert NE (1973) The occurrence of glycolate dehydrogenase and glycolate oxidase in green plants: an evolutionary survey. Plant Physiol 52(4):318–323
Frey W, Stech M (2009) Marchantiophyta, Bryophyta, Anthocerotophyta. In: Frey W (ed) Syllabus of plant families. A. Engler’s syllabus der Pflanzenfamilien, 13th ed., Part 3 Bryophytes and seedless vascular Plants. Gebr. Borntraeger, Stuttgart, pp 1–257
García-Carmona M, Arcenegui V, García-Orenes F, Mataix-Solera J (2020) The role of mosses in soil stability, fertility and microbiology six years after a post-fire salvage logging management. J Environ Manag 262:110287
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gregorczyk A, Raczyńska A (1997) Badania Korelacji miȩdzy metoda Arnona a pomiarami zawartości chlorofilu za pomoca chlorofilometru. Zesz Nauk AR Szczec Rol 68:119–123
Hörtensteiner S (2006) Chlorophyll degradation during senescence. Annu Rev Plant Biol 57:55–77
Kholová J, Hash CT, Kočová M, Vadez V (2011) Does a terminal drought tolerance QTL contribute to differences in ROS scavenging enzymes and photosynthetic pigments in pearl millet exposed to drought? Environ Exp Bot 71:99–106
Kovalchuk I (2010) Multiple roles of radicles in plants. In: Gupta SD (ed) Reactive oxygen species and antioxidants in higher plants. CRC Press, New York, NY, USA, pp 31–44
Luck M (1963) Peroxidase. In: Bergmeyer HV (ed) Methods of Enzymic Analysis. Academic Press, New York, pp 895–897
Makajanma MM, Taufik I, Faizal A (2020) Antioxidant and antibacterial activity of extract from two species of mosses: Leucobryum aduncum and Campylopus schmidii. Biodiversitas 21:2751–2758
Manurung H, Kustiawan W, Kusuma IW, Marjenah (2017) Total flavonoid content and antioxidant activity of tabat Barito (Ficus deltoidea Jack) on different plant organs and ages. J Med Plants Stud 5(6):120–125
Martin CE (1980) Chlorophyll a/b ratios of eleven North Carolina mosses. Bryologist 83:84–87
Martin CE, Churchill SP (1982) Chlorophyll concentrations and a/b ratios in mosses collected from exposed and shaded habitats in Kansas. J Bry 12:297–304
Millan-Almaraz JR, Guevara-Gonzalez RG, Romero-Troncoso R, Osornio-Rios RA, Torres-Pacheco I (2009) Advantages and disadvantages on photosynthesis measurement techniques: A review. Afr J Biotechnol 8:7340–7349
Minibayeva F, Beckett RP (2001) High rates of extracellular superoxide production in bryophytes and lichens, and an oxidative burst in response to rehydration following desiccation. New Phytol 152:333–341
Mukhopadhyay ST, Mitra S, Biswas A, Das N, Poddar-Sarkar M (2013) Screening of antimicrobial and antioxidative potential of selected Eastern Himalayan mosses. Eur J Med Plants 3:422–428
Ochyra R, Lewis Smith RI, Bednarek–Ochyra H (2008) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Oishi Y, Hiura T (2017) Bryophytes as bioindicators of the atmospheric environment in urban-forest landscapes. Landsc Urban Plan 167:348–355
Osawa T (1994) Novel natural antioxidants for utilization in food and biological systems. In: Uritani L, Garcia VV, Mendoza EM (eds) Post Harvest Biochemistry of Plant Food Materials in Tropics. Japan Scientific Societies Press, Tokyo, Japan, pp 241–251
Patiño J, Vanderpoorten A (2018) Bryophyte biogeography. Crit Rev Plant Sci 37:175–209
Pejin B, Bogdanovic-Pristov J, Pejin I, Sabovljevic M (2013) Potential antioxidant activity of the moss Bryum moravicum. Nat Prod Res 27:900–902
Pejin B, Sabovljević A, Soković M, Glamočlija J, Ćirić A, Vujičić M, Sabovljević M (2012) Antimicrobial activity of Rhodobryum ontariense. Hem Ind 66:381–384
Rajalakshmi K, Banu N (2015) Extraction and estimation of chlorophyll from medicinal plants. Intern J Sci Res 4:209–212
Saito K (1975) A monograph of Japanese Pottiaceae (Musci). J Hattori Bot Lab 39:373–537
Singh V, Alam A, Sharma A (2016) Evaluation of phytochemicals, antioxidant and antibacterial activity of Hyophila involuta (Hook.) Jaeg. and Entodon plicatus C. Muell. (Bryophyta) from Rajasthan, India. Int J Sci Res Knowl 4(3):56–63
Spitale D (2016) The interaction between elevational gradient and substratum reveals how bryophytes respond to the climate. J Veget Sci 27:844–853
Vats S, Alam A (2013) Antioxidant activity of Barbula javanica Doz. et Molk.: A relatively unexplored bryophyte. Appl Bot 65:20103–20104
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 46:4113–4117
Xie CF, Lou HX (2009) Secondary metabolites in Bryophytes: An ecological aspect. Chem Biodiv 6(3):303–312
Yayintas OT, Alpaslan D, Yuceer YK, Yilmaz S, Sahiner N (2017) Chemical composition, antimicrobial, antioxidant and anthocyanin activities of mosses (Cinclidotus fontinaloides (Hedw.) P.Beauv. and Palustriella commutata (Hedw.) Ochyra) gathered from Turkey. Nat Prod Res 31:2169–2173
Zander RH (1993) Genera of the Pottiaceae: mosses of harsh environments. Bull Buffalo Soc Nat Sci 32:1–378
Zielewicz W, Wróbel B, Niedbała G (2020) Quantification of chlorophyll and carotene pigments content in mountain melick (Melica nutans L.) in relation to edaphic variables. Forests 11:1197
Author Information
Laboratory No. 14, Department of Botany, University of Rajasthan, Jaipur, India