Physicochemical and functional properties of Garcinia kola Heckel (bitter kola) protein fractions
Osukoya Olukemi Adetutu, Onwuegbunam Chiamaka Laura, Fadugba Abimbola, Salami Salmat Adenike
Research Articles | Published: 08 February, 2024
First Page: 518
Last Page: 527
Views: 3292
Keywords: Seed storage protein, Bitter kola, Antioxidant activities, Functional groups, Dietary protein
Abstract
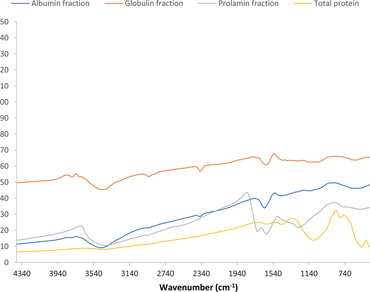
The use of natural compounds as safer substitutes in/as functional foods, dietary supplements, therapeutics and pharmaceuticals is of great interest because of the secondary effects of synthetic compounds. This study investigated the physicochemical, functional and in vitro bioactivities of Garcinia kola seed protein fractions. G. kola seed albumin, globulin and prolamin were extracted with water, saline, and alcohol, respectively. Structural characterization was done by Fourier transform infrared spectroscopy (FTIR). Antioxidant activities (Fe2+-chelating activity and ferric reducing power) and functional properties (solubility, foaming and emulsification) were done using standard procedures. Zone of inhibition test was used to ascertain the antibacterial potentials of the fractions. Prolamin was the predominant protein component of Garcinia kola seeds. FTIR spectrum revealed that the fractions are characterized by Amide I and Amide II bands. The fractions showed significant antioxidant activity, with globulin having the highest activity. Globulin and prolamin fractions exhibited the highest solubility between pH 10–12 and were least soluble at pH 2, while albumin had its lowest solubility at pH 9. High emulsifying activity index was observed for all the fractions, with the highest emulsifying stability index of about 160 min. The fractions also showed no antimicrobial activity, except prolamin, which minimally inhibited Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis. Therefore, Garcinia kola proteins can be effectively used as health-beneficial foods and dietary supplements to improve food nutrition and product diversification.
References
Adebiyi AP, Aluko RE (2011) Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem 128:902–908. https://doi.org/10.1016/j.foodchem.2011.03.116
Barroso da Silva FL et al (2020) Understanding and Controlling Food Protein Structure and function in Foods: perspectives from experiments and computer simulations. Annu Rev Food Sci Technol 11:365–387. https://doi.org/10.1146/annurev-food-032519-051640
Buba CI, Okhale SE, Muazzam I (2016) Garcinia kola: the phytochemistry, pharmacology and therapeutic applications. Int J Pharmacognosy 3:67–81
Chalamaiah M, Jyothirmayi T, Diwan PV, Dinesh Kumar B (2015) Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J Food Sci Technol 52:5817–5825. https://doi.org/10.1007/s13197-015-1714-6
Dada SO, Ehie GC, Osukoya OA, Anadozie SO, Adewale OB, Kuku A (2023) In vitro antioxidant and anti-inflammatory properties of Artocarpus altilis (Parkinson) Fosberg (seedless breadfruit) fruit pulp protein hydrolysates. Sci Rep 13:1493. https://doi.org/10.1038/s41598-023-28684-z
Dash P, Ghosh G (2017a) Fractionation, amino acid profiles, antimicrobial and free radical scavenging activities of Citrullus lanatus seed protein. Nat Prod Res 31:2945–2947. https://doi.org/10.1080/14786419.2017.1305385
Dash P, Ghosh G (2017b) Proteolytic and antioxidant activity of protein fractions of seeds of Cucurbita moschata. Food Bioscience 18:1–8. https://doi.org/10.1016/j.fbio.2016.12.004
De Meutter J, Goormaghtigh E (2021a) Amino acid side chain contribution to protein FTIR spectra: impact on secondary structure evaluation. Eur Biophys J 50:641–651. https://doi.org/10.1007/s00249-021-01507-7
De Meutter J, Goormaghtigh E (2021b) Evaluation of protein secondary structure from FTIR Spectra improved after partial deuteration. Eur Biophys J 50:613–628. https://doi.org/10.1007/s00249-021-01502-y
Dogara AM, Hamad SW, Hama HA, Bradosty SW, Kayfi S, Al-Rawi SS, Lema AA (2022) Biological Evaluation of Garcinia kola Heckel. Advances in Pharmacological and Pharmaceutical Sciences 2022:3837965. https://doi.org/10.1155/2022/3837965
Farombi EO, Adepoju BF, Ola-Davies OE, Emerole GO (2005) Chemoprevention of aflatoxin B1-induced genotoxicity and hepatic oxidative damage in rats by kolaviron, a natural biflavonoid of Garcinia kola seeds. Eur J Cancer Prev:207–214
Guo X, Yao H (2006) Fractionation and characterization of tartary buckwheat flour proteins. Food Chem 98:90–94. https://doi.org/10.1016/j.foodchem.2005.05.055
Henchion M, Hayes M, Mullen AM, Fenelon M, Tiwari B (2017) Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods 6. https://doi.org/10.3390/foods6070053
Idowu AO, Alashi AM, Nwachukwu ID, Fagbemi TN, Aluko RE (2021) Functional properties of sesame (Sesamum indicum Linn) seed protein fractions. Food Prod Process Nutr 3:4. https://doi.org/10.1186/s43014-020-00047-5
Jabs A (2005) Determination of secondary structure in proteins by fourier transform infrared spectroscopy (FTIR). Jena Library of Biologica Macromolecules
Kruger NJ (2009) The Bradford Method for protein quantitation. In: Walker JM (ed) The protein protocols handbook. Humana Press, Totowa, NJ, pp 17–24. https://doi.org/10.1007/978-1-59745-198-7_4
Nalinanon S, Benjakul S, Kishimura H, Shahidi F (2011) Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem 124:1354–1362
Orona-Tamayo D, Valverde ME, Nieto-Rendón B, Paredes-López O (2015) Inhibitory activity of Chia (Salvia hispanica L.) protein fractions against angiotensin I-converting enzyme and antioxidant capacity. LWT - Food Science and Technology 64:236–242. https://doi.org/10.1016/j.lwt.2015.05.033
Osborne TB (1924) The vegetable proteins. Longmans, Green and Company
Osukoya OA, Adewale OB, Falade AE, Afolabi OB, Awe JO, Obafemi TO, Kuku A (2021) Antioxidant and antibacterial properties of Monodora myristica (Calabash Nutmeg) seed protein hydrolysates. J Food Meas Charact 15:2854–2864. https://doi.org/10.1007/s11694-021-00871-4
Osukoya OA, Cheke A, Adewale OB, Awe JO, Olasehinde O (2019) In vitro antioxidant and analgesic properties of methanolic extracts of the fruits of Xylopia aethiopica on formalin-induced nociception. Nigerian J Environ Health 2:57–63
Osukoya OA et al (2022) Some functional properties, antioxidant and bactericidal activities of Garcinia kola Heckel protein hydrolysates: in vitro. https://doi.org/10.1007/s42535-022-00526-9. Vegetos
Perez C (1990) Antibiotic assay by agar-well diffusion method. Acta Biologiae Et Medicine Experimentalis 15:113–115
Salami SA, Osukoya OA, Adewale OB, Odekanyin O, Obafemi TO, Kuku A (2023) Bioactivities of Garcinia kola enzymatic hydrolysates at different enzyme–substrate ratios. AMB Express 13:78. https://doi.org/10.1186/s13568-023-01583-2
Scopes RK (1993) Protein purification: principles and practice. Springer Science & Business Media
Scopes RK, Smith JA (2006) Analysis of proteins. Curr Protoc Mol Biol 76. https://doi.org/10.1002/0471142727.mb1000s76
Shewry PR, Casey R (1999) Seed proteins. In: Shewry PR, Casey R (eds) Seed proteins. Springer Netherlands, Dordrecht, pp 1–10. https://doi.org/10.1007/978-94-011-4431-5_1
Smith DM (2017) Protein separation and characterization procedures. In: Nielsen SS (ed) Food Analysis. Springer International Publishing, Cham, pp 431–453. https://doi.org/10.1007/978-3-319-45776-5_24
Song K (2017) 4 - Interphase characterization in rubber nanocomposites. In: Thomas S, Maria HJ (eds) Progress in Rubber Nanocomposites. Woodhead Publishing, pp 115–152. https://doi.org/10.1016/B978-0-08-100409-8.00004-8
Wilde PJ (2000) Interfaces: their role in foam and emulsion behaviour. Curr Opin Colloid Interface Sci 5:176–181. https://doi.org/10.1016/S1359-0294(00)00056-X
Yakubu CM, Sharma R, Sharma S, Singh B (2022) Influence of alkaline fermentation time on in vitro nutrient digestibility, bio- & techno-functionality, secondary protein structure and macromolecular morphology of Locust bean (Parkia biglobosa) flour. LWT 161:113295. https://doi.org/10.1016/j.lwt.2022.113295
Yuan H, Wang H, Wang L, Chai L, Tian C (2017) Nutritional evaluation and functional properties of the antioxidant polypeptide from Zanthoxylum Bungeanum Maxim seeds kernel protein hydrolysate. CyTA-Journal of Food 15:425–432. https://doi.org/10.1080/19476337.2017.1288171
Zayas JF (1997) Functionality of proteins in food. Springer science & business media
Zeng H-Y, Cai L-H, Cai X-L, Wang Y-J, Li Y-Q (2011) Structure characterization of protein fractions from lotus (Nelumbo nucifera) seed. J Mol Struct 1001:139–144. https://doi.org/10.1016/j.molstruc.2011.06.031
Jeevitha K, Mohana PK, Khora SS (2014) Antioxidant activity of fish protein hydrolysates from Sardinella longiceps. Int J Drug Dev Res 6:137–145. https://www.itmedicalteam.pl/articles/antioxidant-activity-of-fish-protein-hydrolysates-from-sardinellalongiceps-101330.html
Li Y, Zheng Y, Zhang Y, Xu J, Gao G (2018) Antioxidant activity of coconut (Cocos nucifera L.) protein fractions. Molecules 23. https://doi.org/10.3390/molecules23030707
Author Information
Department of Chemical Sciences, Afe Babalola University, Ado-Ekiti, Nigeria