Optimization of the process for obtaining Morinda royoc crude extract bioactive against phythopathogens
Research Articles | Published: 20 July, 2023
First Page: 1336
Last Page: 1345
Views: 3032
Keywords: Anthraquinones, Antifungal activity, Extraction process
Abstract
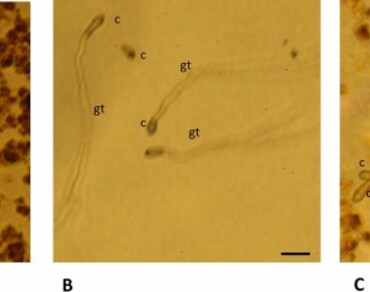
Currently, there is an increased search for alternatives to reduce the excessive use of synthetic pesticides in control of pests and diseases in plants. Use of natural plant products is considered an efficient way to reduce the use of these harmful products and achieve the control of phytopathogens in agricultural production. In this sense, Morinda royoc L. is known for the high content of anthraquinones (AQs) in its roots, which have been studied for their distinct bioactivity. Therefore, the aim of this study was to optimize the extraction procedure to obtain crude extracts of M. royoc roots rich in bioactive AQs and to evaluate the antifungal activity in vitro against the fungi Botrytis cinerea, Neofabraea alba and Venturia inaequalis. The extraction procedure by maceration was optimized in terms of extraction time, hydroethanolic mixture and temperature parameters; in vitro inhibitory effect of the obtained extract on mycelial growth and conidial germination of three fungi was evaluated. M. royoc root extract was optimized at 42.31 mg AQs/g dry weight; and its antifungal potential was demonstrated by complete inhibition of conidial germination of B. cinerea, N. alba, and V. inaequalis at concentrations of 4.8 to 2.4 mg/ml; 4.8 to 0.15 mg/ml; and 4.8 to 0.3 mg/ml, respectively. Based on the efficacy of the extract as an in vitro antifungal control, it could be used as a promising product for crop protection.
References
Ahmadu T, Ahmad K, Ismail SI, Rashed O, Asib N, Omar D (2021) Antifungal efficacy of Moringa oleifera leaf and seed extracts against Botrytis cinerea causing gray mold disease of tomato (Solanum lycopersicum L.). Braz J Biol 81(4):1007–22. https://doi.org/10.1590/1519-6984.233173
Alam P, Noman OM, Herqash RN, Almarfadi OM, Akhtar A, Alqahtani AS (2022) Efficient extraction of an anthraquinone physcion using response surface methodology (RSM) optimized ultrasound-assisted extraction method from aerial parts of Senna occidentalis and analysis by HPLC-UV. Separations 9(142):1–12. https://doi.org/10.3390/separations9060142
Apolonio I, Franco O, Salgado M, Aquino J (2017) In vitro inhibition of Botrytis cinerea with extracts of wild grapevine (Vitis Spp.) leaves. Mex J PhytoPathol 35(2):170–85. https://doi.org/10.18781/R.MEX.FIT.1611-1
Balint J, Nagy S, Thiesz R, Nyaradi I, Baloga D (2014) Using plant extracts to reduce asexual reproduction of apple scab (Venturia inaequalis). Turk J Agric For 38:91–98. https://doi.org/10.3906/tar-1302-17
Bengtsson M, Wulff E, Lyngs H, Pham A, Lübeck M, Hockenhull J (2009) Comparative studies on the effect of a yucca extract and acibenzolar-s-methyl (asm) on inhibition of Venturia inaequalis in apple leaves. Eur J Plant Pathol. https://doi.org/10.1007/s10658-008-9405-z
Borroto J, Coll J, Rivas M, Blanco M, Concepción O, Tandrón YA, Trujillo R (2008) Anthraquinones from in vitro root culture of Morinda royoc L. Plant Cell Tiss Organ Cult 94:181–187. https://doi.org/10.1007/s11240-008-9403-z
Borroto J, Salazar R, Pérez A, Quiros Y, Hernandez M, Waksman N, Trujillo R (2010) Antimicrobial activity of the dichloromethane extract from in vitro cultured roots of Morinda royoc and its main constituents. Nat Prod Commun 5(5):809–810
Bustillo AJ, Aleu J, Hernández R, Collado IG (2003) Biotransformation of the fungistatic compound (R)-(+)-1-(4-chlorophenyl) propan-1-ol by Botrytis cinerea. J Mol Catal B Enzym 21(21):267–271
Chan C, Yusoff R, Ngoh G, Wai-lee KF (2011) Microwave-assisted extractions of active ingredients from plants. J Chromatogr A 1218(37):6213–6225. https://doi.org/10.1016/j.chroma.2011.07.040
Cid G, Linares C, Rivas M, Quiñones J, Capdesuñer Y (2020) Antimicrobial activity of bioactive crude extracts of Morinda royoc L. roots grown in Cuba. Revista De Protección Vegetal 35(1):1–13
Daniel C, Lennox C, Vries F (2015) In vitro effects of garlic extracts on pathogenic fungi Botrytis cinerea, Penicillium expansum and Neofabraea alba. S Afr J Sci 111(7):1–8. https://doi.org/10.17159/sajs.2015/20140240
Diaz-Muñoz G, Miranda IL, Sartori SK, de Rezende DC, Diaz MA (2018) Anthraquinones: an overview. Stud Nat Prod Chem 58:313–338
Doolotkeldieva T, Bobusheva S (2017) Scab disease caused by Venturia inaequalis on apple trees in kyrgyzstan and biological agents to control this disease. Adv Microbiol 7:450–466. https://doi.org/10.4236/aim.2017.76035
Drenker C, El Mazouar D, Bücker G, Weißhaupt S, Wienke E, Koch E, Kunz S, Reineke A, Rondot Y, Linkies A (2023) Characterization of a disease-suppressive isolate of lysobacter enzymogenes with broad antagonistic activity against bacterial, oomycetal and fungal pathogens in different crops. Plants 12(3):682. https://doi.org/10.3390/plants12030682
Emara AR, Ibrahim HM, Masoud SA (2021) The role of storage on Mancozeb fungicide formulations and their antifungal activity against Fusarium oxysporium and Rhizoctonia solani. Arab J Chem 14(10):103322. https://doi.org/10.1016/j.arabjc.2021.103322
Glavnik V, Vovk I (2020) Extraction of anthraquinones from japanese knotweed rhizomes and their analyses by high performance thin-layer chromatography and mass spectrometry. Plants 1753:1–16. https://doi.org/10.3390/plants9121753
Han YS, van der Heijden R, Verpoorte R (2002) Improved anthraquinone accumulation in cell cultures of Cinchona Robusta by feeding of biosynthetic precursors and inhibitors. Biotechnol Lett 24:705–710. https://doi.org/10.1023/A:1015286117297
Hemwimon S, Pavasant P, Shotipruk A (2007) Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep Purif Technol 54(1):44–50. https://doi.org/10.1016/j.seppur.2006.08.014
Hernández J, Volpato G (2004) Herbal Mixtures in the traditional medicine of eastern Cuba. J Ethnopharmacol 90:293–316. https://doi.org/10.1016/j.jep.2003.10.012
Jamar L, Cavelier M, Lateur M (2010) Primary scab control using a “during-infection” spray timing and the effect on fruit quality and yield in organic apple production. Biotechnol Agron Soc Environ 14(3):423–439
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18:2328–2375. https://doi.org/10.3390/molecules18022328
Kunz S, Mögel G, Hinze M, Volk F (2008) Control of apple scab by curative applications of biocontrol agents. In: Proceedings of the Ecofruit-13th international conference on cultivation technique and phytopathological problems in organic fruit-growing, Weinsberg, Germany. Fördergemeinschaft Ökologischer Obstbau e.V., Weinsberg, Germany, p 62–67
Levin DA (1976) The chemical defenses of plants to pathogens and herbivores. Annu Rev Ecol Evolut Syst 7:121–159
Mendoza LM, Aturana R, Cardona W, Delgado C, Garcia T, Lagos C, Cotoras M (2005) In vitro sensitivity of Botrytis cinerea to anthraquinone and anthrahydroquinone derivatives. J Agric Food Chem 53:10080–10084
Mesa VAM, Marín P, Ocampo O, Calle J, Monsalve Z (2019) Fungicidas a partir de extractos vegetales: una alternativa en el manejo integrado de hongos fitopatógenos. RIA Revista De Investigaciones Agropecuarias 45(1):23–30
Mishra P, Tripathi A, Dikshit A, Pandey A (2020) Insecticides derived from natural products: diversity and potential applications. Agriculture. https://doi.org/10.1007/978-981-15-3024-1
Mohideen FI, Kwan DH (2023) A biphasic glycosyltransferase high-throughput screen identifies novel anthraquinone glycosides in the diversification of phenolic natural products. J Biol Chem 299(3):102931. https://doi.org/10.1016/j.jbc.2023.102931
Neri F, Mari M, Brigati S, Bertolini P (2009) Postharvest biology and technology control of Neofabraea alba by plant volatile compounds and hot water. Postharvest Biol Technol 51:425–430. https://doi.org/10.1016/j.postharvbio.2008.08.006
Paunović D, Mitić S, Kostić DA, Mitić MN, Stojanović J, Pavlović BT (2014) Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols from barley. Adv Technol 3(2):58–63. https://doi.org/10.5937/savteh1402058P
Pérez L, Mendoza J, Quirós Y, Leiva M, Martinez Montero M, Acosta M, Ferrer A, Trujillo R, Pérez AT (2022) Antifungal activity of Mosiera bullata (Britton & P. Wilson) extract against phytopathogenic fungi. Vegetos. https://doi.org/10.1007/s42535-022-00540-x
Porsche FM, Molitor D, Beyer M, Charton S, Andre C, Andreas K (2018) Antifungal activity of saponins from the fruit pericarp of sapindus mukorossi against Venturia Inaequalis and Botrytis cinerea. Plant Dis 102:991–1000. https://doi.org/10.1094/PDIS-06-17-0906-RE
Quintal-Novelo C, Valencia L, Chávez A, Rangel J, Moo R (2022) A Morinda royoc root extract and fractions exhibit antigiardial activity without affecting cell viability. Iranian J Parasitol 17(2):259. https://doi.org/10.18502/ijpa.v17i2.9544
Quitério E, Grosso C, Ferraz R, Delerue-Matos C, Soares C (2022) A critical comparison of the advanced extraction techniques applied to obtain health-promoting compounds from seaweeds. Mar Drugs 20(677):2–40. https://doi.org/10.3390/md20110677
Rajbhar K, Dawda H, Mukundan U (2015) Polyphenols: methods of extraction. Sci Rev Chem Commun 5(1):1–6
Ramos V, Bocalandros C, Riquelme S, Sanhueza V, Aspé E, Roeckel M, Fernández K (2013) Effect of the bench scale extraction conditions on Pinus radiata bark extract yield, antioxid ant properties and composition. Maderas. Ciencia y Tecnoiogia 15(1):31–44. https://doi.org/10.4067/S0718-221X2013005000003
Reyes C, Rodríguez S, Montelongo C, Gim C, Jim I, Cabrera R, Bazzochi I (2022) Antifungal potential of canarian plant extracts against. Plants 11(2988):1–14. https://doi.org/10.3390/plants11212988
Sakulpanich A, Gritsanapan W (2008) Extraction method for high content of anthraquinones from Cassia fistula pods. J Health Res 22(4):167–172
Saravanakumar K, Lu Z, Xia H, Wang M, Sun J, Wang S, Wang Q, Li Y, Chen J (2018) Triggering the biocontrol of Botrytis cinerea by Trichoderma harzianum through inhibition of pathogenicity and virulence related proteins. Front Agric Sci Eng 5(2):271–79. https://doi.org/10.15302/J-FASE-2018214
Schulte U, EI-ShagiZenk HM (1984) Optimization of 19 Rubiaceae species in cell culture for the production of anthraquinones. Plant Cell Rep 31:51–54
Shabana Y, El-Boray M, Mustafa M, Al-Juboori G (2015) Antifungal activity of plant extracts, essential oils, and microbial culture filtrates against Botrytis cinerea in vitro. J Plant Prot Res 6(9):1297–1311
Soto M, Rosales M (2016) Effect of solvent and solvent-to-solid ratio on the phenolic extraction and the antioxidant capacity of extracts from Pinus durangensis and Quercus sideroxyla bark. Maderas. Ciencia y Tecnología 18(4):701–714. https://doi.org/10.4067/S0718-221X2016005000061
Šunjka D, Mechora Š (2022) An alternative source of biopesticides and improvement in their formulation recent advances. Plants 11(3172):1–13. https://doi.org/10.3390/plants11223172
Takó M, Kerekes EB, Zambrano C, Kotogán A, Papp T, Krisch J, Vágvölgyi C (2020) Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food contaminating microorganisms. Antioxidants 9:165. https://doi.org/10.3390/antiox9020165
Tamm L, Thuerig B, Apostolov S, Blogg H, Borgo E, Corneo P, Fittje S (2022) Use of copper-based fungicides in organic agriculture in twelve european countries. Agronomy 12(673):1–21. https://doi.org/10.3390/agronomy12030673
Temiyaputra W, Suebsanga T, Yajom K, Piyaworanon S, Leksawasdi N (2015) Influences of anthraquinone extraction techniques from Morinda sp. on extraction efficiency. Kasetsart J Nat Sci 42(5):118–126
Thach NA (2022) Investigation of the effects of extraction temperature and time on bioactive compounds content from garlic (Allium sativum L.) husk. Front Sustain Food Syst 6:1–6. https://doi.org/10.3389/fsufs.2022.1004281
Thiesz R, Balogi A, Ferencz L, Albert J (2007) Venturia inaequalis Cooke. The effects of plant extracts on apple scab under laboratory conditions. Roum Biotechnol Lett 12(4):3295–3330
Vio-Michaelis S, Apablaza G, Gómez M, Peña R, Montenegro G (2012) Antifungal activity of three chilean plant extracts on Botrytis cinerea. Bot Sci 90(2):179–183
Xu L, Li X, Cui Y, Pang M, Wang F, Qi J (2017) Antibacterial activity of anthraquinone from aloe on spiced pig head. IOP Conf Ser Mater Sci Eng 275:1–6. https://doi.org/10.1088/1757-899X/275/1/012014
Yang JY, Chung KR, Huang JW (2020) A combined effect of Bacillus sp, tobacco extracts and plant oils on the control of cruciferous vegetable anthracnose. Arch Phytopathol Plant Prot 53(1–2):48–69. https://doi.org/10.1080/03235408.2020.1717253
Author Information
Natural Products Department, Centro de Bioplantas, Universidad de Ciego de Ávila Máximo Gómez Báez, Ciego de Ávila, Cuba