Nutritional, phenol content and antioxidant activity of edible sprouts of commonly occurring plants
Research Articles | Published: 04 March, 2024
First Page: 2131
Last Page: 2137
Views: 3145
Keywords: Micro sprouts, Antioxidant activity, Phenolic substance, Protein, Ascorbic acid
Abstract
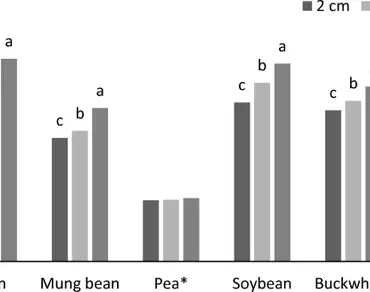
The present investigation was carried out to analyse the nutritional aspects along with the phenol profile and antioxidant activity of four edible sprouts of commonly occurring legumes species, buckwheat and quinoa plants. Seeds of beans, mung beans, soybeans, peas, quinoa and buckwheat as a seed material were used in this study. The aim of the study is to determine the nutritional, phenolic and antioxidant content of some legumes and different plant species sprouted in different sizes. In the study, sprouting was continued until it reached 2 cm, 4 cm and 6 cm, then the sprouts were analyzed. In the research, it was determined that the relative humidity, crude protein, ascorbic acid, total soluble sugar and total phenolic substance content increased in the studied species depending on the sprout size. Antioxidant activity increased with increased sprouts size, in beans, peas, mung beans and buckwheat, and decreased in soybean and quinoa species. As a result of the study, the size of the micro sprouts that should be consumed according to the plant species also changes. The conclusion can be drawn from the study that the length of 6 cm size sprouts of bean, mung bean and buckwheat species, 4 cm soybean, and in quinoa and peas of all sizes of sprouts exhibits the maximum nutritional and functional properties in the study which suggested the potential health benefit.
References
Bertamini M, Zulini L, Muthuchelian K, Nedunchezhian N (2006) Effect of water deficit on photosynthetic and other physiological responses in grapevine (Vitis vinifera L. cv. Riesling) plants. Photosynthetica 44(1):151–154
Cevallos-Casals BA, Cisneros-Zevallos L (2010) Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem 119(4):1485–1490. https://doi.org/10.1016/j.foodchem.2009.09.030
González L, González-Vilar M (2001) Determination of relative water content. In: Reigosa Roger MJ (ed) Handbook of plant ecophysiology techniques, 1st edn. Kluwer Academic Publishers, Dordrecht, pp 211–217
Gündüz GA (2021). What is micro hercule? In: Micro Hercules. Available via DIALOG. https://www.dogaclama.com/ic/mikro-herkul-nedir-. Accessed 22 Feb 2021
Huang X, Cai W, Xu B (2014) Kinetic changes of nutrients and antioxidant capacities of germinated soybean (Glycine max L.) and mung bean (Vigna radiata L.) with germination time. Food Chem 143:268–276
Kacar B, Inal A (2010) Plant analysis. Nobel Publication Distribution, Ankara
Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Games EJC, Mehta RG, Kinghorn AD, Pezzuto JM (1998) Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen 1:35–46
Li YC, Qian H, Sun XL, Cui Y, Wang HY, Du C, Xia XH (2014) The effects of germination on chemical composition of peanut seed. Food Sci Technol Res 20(4):883–889. https://doi.org/10.3136/fstr.20.883
Masood T, Shah HU, Zeb A (2014) Effect of sprouting time on proximate composition and ascorbic acid level of mung bean (Vigna radiata L.) and chickpea (Cicer arietinum L.) seeds. J Anim Plant Sci 24(3):850–859
Nonogaki H, Bassel GW, Bewley JD (2010) Germination still a mystery. Plant Sci 179(6):574–581. https://doi.org/10.1016/j.plantsci.2010.02.010
Pearson D, Churchil AA (1970) The chemical analyses of foods. Gloucester Place, Longman Group Ltd, Harlow
Plaza P, Usall J, Teixidó N, Vinas I (2003) Effect of water activity and temperature on germination and growth of Penicillium digitatum, Penicillium italicum and Geotrichum candidum. J Appl Microbiol 94(4):549–554. https://doi.org/10.1046/j.1365-2672.2003.01909.x
Pranzik W, Mundingler N, Kogler A, Pelzi B, Huber A, Wollendorfer M (1999) Molecular back ground of technological properties of selected starches. Starch-Stärke 51(6):197–211. https://doi.org/10.1002/(SICI)1521-379X(199906)51:6%3c197::AID-STAR197%3e3.0.CO;2-K
Rodríguez C, Frias J, Vidal-Valverde C, Hernández A (2008) Correlations between some nitrogen fractions, lysine, histidine, tyrosine, and ornithine contents during the germination of peas, beans, and lentils. Food Chem 108(1):245–252. https://doi.org/10.1016/j.foodchem.2007.10.073
Ross KA, Beta T, Arntfield SD (2009) A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem 113(1):336–344. https://doi.org/10.1016/j.foodchem.2008.07.064
Sattar D, Ali TM, Hasnain A (2017) Effect of nongerminated and germinated legumes on antioxidant, functional, and sensory characteristics of rice puddings. Cereal Chem 94(3):417–423. https://doi.org/10.1094/CCHEM-04-16-0103-R
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. AJEV 16:144–158
Ti H, Zhang R, Zhang M, Li Q, Wei Z, Zhang Y, Ma Y (2014) Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem 161:337–344. https://doi.org/10.1016/j.foodchem.2014.04.024
Urbano G, López-Jurado M, Aranda C, Vilchez A, Cabrera L, Porres JM, Aranda P (2006) Evaluation of zinc and magnesium bioavailability from pea (Pisum sativum L.) sprouts. Effect of illumination and different germination periods. Int J Food Sci. 41(6):618–626. https://doi.org/10.1111/j.1365-2621.2005.01107.x
Vidal-Valverde C, Frias J, Sierra I, Blazquez I, Lambein F, Kuo YH (2002) New functional legume foods by germination: effect on the nutritive value of beans, lentils and peas. Eur Food Res Technol 215(6):472–477. https://doi.org/10.1007/s00217-002-0602-2
Winarsi H, Septiana AT, Wulandari SP (2020) Germination improves sensory, phenolic, protein content and anti-inflammatory properties of red kidney bean (Phaseolus vulgaris L.) sprouts milk. Food Research 4(6):1921–1928
Wongsiri S, Ohshima T, Duangmal K (2015) Chemical composition, amino acid profile and antioxidant activities of germinated mung beans (Vigna radiata). J Food Process Preserv 39(6):1956–1964. https://doi.org/10.1111/jfpp.12434
Xue Z, Wang C, Zhai L, Yu W, Chang H, Kou X, Zhou F (2016) Bioactive compounds and antioxidant activity of mung bean (Vigna radiata L.), soybean (Glycine max L.) and black bean (Phaseolus vulgaris L.) during the germination process. Czech J Food Sci. 34(1):68–78. https://doi.org/10.17221/434/2015-CJFS
Zielinski H (2002) Peroxyl radical-trapping capacity of germinated legume seeds. Food Nahrung 46(2):100–104. https://doi.org/10.1002/1521-3803(20020301)46:2%3c100::AID-FOOD100%3e3.0.CO;2-Z
Author Information
Isparta University of Applied Sciences, Isparta, Turkey