Leaf explant based direct and indirect regeneration and SCoT marker assisted genetic fidelity analysis of endemic taxon Corynandra chelidonii var. pallae
Sirangi Subhash, Sandhya Dulam, Rohela Gulab Khan, Shekhawat Mahipal S., Ajmeera Ragan, Raju Vatsavaya S.
Research Articles | Published: 24 November, 2023
First Page: 2588
Last Page: 2599
Views: 3478
Keywords: Callus induction, n Corynandran , Genetic homogeneity, Organogenesis, SCoT primers
Abstract
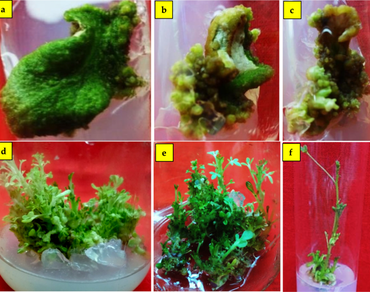
Corynandra chelidonii var. pallae (Family: Cleomaceae) is an endemic and rare taxon with declining populations over the past few years. An attempt was made to develop a reliable protocol for its micropropagation through leaf explant-based direct and indirect organogenesis. The highest mean number of shoots (13.46 ± 0.29 per explant) and shoot length (1.06 ± 0.0213 cm) were achieved on Murashige and Skoog (MS, 1962) medium supplemented with 2 mg L−1 of 6-benzylaminopurine (BAP) during direct regeneration from the leaf segment explants. For indirect organogenesis, callus was induced first in a maximum amount on MS medium supplemented with 2 mg L−1 of 2,4-dichlorophenoxyacetic acid (2,4-D) from the leaf segments. Indirect regeneration with the highest mean number of shoots (6.08 ± 0.23) and shoot length (2.06 ± 0.007 cm) were obtained on MS medium with 2 mg L−1 BAP and 0.5 mg L−1 naphthalene acetic acid (NAA). The regenerated shoots were rooted successfully (100%) on half-strength MS medium supplemented with 1 mg L−1 of indole-3-butyric acid (IBA). The regenerated plantlets were acclimatized in pots possessing cocopeat. The acclimatized plantlets showed a survival rate of 60% under field conditions. The genetic diversity of regenerated plantlets and the mother plant were evaluated using Start Codon Targeted (SCoT) markers. It is of much interest that the DNA bands were monomorphic across all samples.
References
Abdullah W, Elsayed WM, Abdelshafeek KA, Nazif NM, Singab ANB (2016) Chemical constituents and biological activities of Cleome genus: a brief review. Int J Pharma Phytochem Res 8(5):777–787
Agha HM, Radzun KA, Sidik NJ, Jawad AH (2022) Callus induction of fenugreek Trigonella foenum-graecum via auxin combined with cytokinins hormones, and assessment of toxicity via brine shrimp assay. J Asian Sci Res 12(1):12–27
Ahmad N, Faisal M, Anis M, Aref IM (2010) In vitro callus induction and plant regeneration from leaf explants of Ruta graveolens L. S Afr J Bot 76(3):597–600
Anburaj J, Singh CR, Kuberan T, Sundaravadivelan C, Kumar P (2011) Effects of plant growth regulators on callus induction from leaf explants of Cleome viscosa. Res J Pharm Biol Chem Sci 2(2):576–583
Bakhtiar Z, Mirjalili MH, Sonboli A (2016) In vitro callus induction and micropropagation of Thymus persicus (Lamiaceae), an endangered medicinal plant. Crop Breed Appl Biot 16:48–54
Bala R, Beniwal VS, Laura JS (2015) An efficient and reproducible indirect shoot regeneration from female leaf explants of Simmondsia chinensis, a liquid-wax producing shrub. Phys Mol Biol Plants 21(2):293–299
Basak H, Biswas BK, Azad MAK, Arifuzzaman M, Sharmeen F (2012) Micropropagation of Mustard (Brassica spp.) from leaf explants. Thai J Agric Sci 45(2):75–81
Bashan Y, de Bashan LE, Prabhu SR, Hernandez JP (2014) Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil 378(1):1–33
Batool TS, Shoaib MS, Baloch PA, Akhtar J, Ali QM (2020) Effects of different potting media during hardening of tissue-cultured-raised-banana plants. Int J Biol Biotechnol 17:83–86
Chi VV, Hop T (2002) Cˆay cỏ c´o ´ıch [Vietnam useful plants]. Vietnam Education Publishing House, Hanoi
Cochrane TS, Iltis HH (2014) Studies in the Cleomaceae VII: five new combinations in Corynandra, an earlier name for Arivela. Novon J Bot Nom 23(1):21–26
Das S, Jha LK (2018) Effect of different rooting media on root proliferation of Taxus baccata L. stem cuttings. Curt Agric Res J 6(1):95
Doyle J (1991) DNA protocols for plants. In: Hewitt GM, Johnston AWB, Young JPW (eds) Molecular techniques in taxonomy. Springer, Berlin, pp 283–293. https://doi.org/10.1007/978-3-642-83962-7_18
El zayat MAS, Ali MES, Amar MH (2020) A systematic revision of Capparaceae and Cleomaceae in Egypt: an evaluation of the generic delimitations of Capparis and Cleome using ecological and genetic diversity. J Genet Eng Biotechnol 18:58. https://doi.org/10.1186/s43141-020-00069-z
Feodorova TA, Voznesenskaya EV, Edwards GE, Roalson EH (2010) Biogeographic patterns of diversification and the origins of C4 in Cleome (Cleomaceae). Syst Bot 35(4):811–826
Grubben GJH (2004) Plant resources of tropical Africa: vegetables (PROTA 2), vol 2. PROTA
Gupta R, Chakrabarty SK (2013) Gibberellic acid in plant: still a mystery unresolved. Plant Sign Behav 8(9):e25504
Hadi A, Singh S, Rafiq S, Nawchoo IA, Wagay NA, Mahmoud EA, Elansary HO (2022) In vitro propagation of Aconitum violaceum Jacq. ex Stapf through seed culture and somatic embryogenesis. Horticulture 8(7):599
Hamdeni I, Louhaichi M, Slim S, Boulila A, Bettaieb T (2022) Incorporation of organic growth additives to enhance in vitro tissue culture for producing genetically stable plants. Plants 11(22):3087
Horvath D (2009) Common mechanisms regulate flowering and dormancy. Plant Sci 177(6):523–531
Huxley A (ed) (1992) New RHS dictionary of gardening 1: 652–653. The Macmillan Press Ltd., London
Jogam P, Sandhya D, Shekhawat MS, Alok A, Manokari M, Abbagani S, Allini VR (2020) Genetic stability analysis using DNA barcoding and molecular markers and foliar micro-morphological analysis of in vitro regenerated and in vivo grown plants of Artemisia vulgaris L. Indian Crops Prod 151:112476
Juarez-Vazquez MDC, Jimenez-Arellanes MA, Jimenez-Arellanes MA (2019) Phytochemical investigation, anti-inflammatory and antinociceptive activities from some species of Cleomaceae family: a systematic review. Adv Med Plant Res 7(4):107–128
Kahrizi D, Soorni J (2013) Study on shoot regeneration and somatic embryogenesis in cumin (Cuminum cyminum L.) landraces. Biha Biol 7(1):37–41
Kiran SK, Galatali S, Yeniocak S, Ozkaya DE, Mercan T, Guldag S, Celik O, Ghafoor NA, Kaya E (2021) Investigation of modified WPM medium for the best meristem proliferation of Corylus avellana L. Adv Hort Sci 35(3):285–292
Kirtikar KR, Basu BD (1935) Indian MEDICINAL PLANts 3: 2171–2172. Periodical Expert Book Agency, New Delhi
Kumar A, Goyal SC, Lata C, Sharma N, Dhansu P, Parshad J (2017) Rapid, efficient direct and indirect regeneration protocol of Dioscorea deltoidea Wall. Nat Acad Sci Lett 40(4):237–240
Manokari M, Priyadharshini S, Cokulraj M, Jayaprakash K, Dey A, Faisal M, Alatar AA, Alok A, Shekhawat MS (2022) Polyethylene glycol mediated improved shoot proliferation, foliar morpho-anatomy, and rooting of micropropagated shoots of Spathoglottis plicata Blume. S Afr J Bot 146:897–904
Marshall DM, Muhaidat R, Brown NJ, Liu Z, Stanley S, Griffiths H, Sage RF, Hibberd JM (2007) Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis. Plant J 51(5):886–896
Mirani AA, Abul-Soad AA, Markhand GS (2017) Effect of different substrates on survival and growth of transplanted orchids (Dendrobium nobile cv.) into net house. Int J Hortic Fl 5(4):310–317
Mood K, Jogam P, Sirikonda A, Shekhawat MS, Rohela GK, Manokari M, Allini VR (2022) Micropropagation, morpho-anatomical characterization, and genetic stability studies in Lippia javanica (Burm. f.) Spreng: a multipurpose medicinal plant. Plant Cell Tissue Org Cult 150:427–437
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nguyen PD, Sayagh C, Borie N, Lavaud C (2017) Antiradical flavonol glycosides from the aerial parts of Cleome chelidonii L. f. Phytochemistry 142:30–37
Nongdam P, Tikendra L (2014) Establishment of an efficient in vitro regeneration protocol for rapid and mass propagation of Dendrobium chrysotoxum Lindl. using seed culture. Sci World J 2014:1–8
Ozudogru EA, Kaya E, Kirdok E, Issever-Ozturk S (2011) In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cell Dev Biol-Plant 47:309–320
Ozudogru EA, Kaya E, Lambardi M (2013) In vitro propagation of peanut (Arachis hypogaea L.) by shoot tip culture. In: Protocols for micropropagation of selected economically-important horticultural plants, pp 77–87
Patel B, Gami B, Patel N, Bariya V (2015) One step pre-hardening micropropagation of Bambusa balcooa Roxb. J Phytol 7:1–9
Pendli S, Rohela GK, Jogam P, Bylla P, Korra R, Thammidala C (2019) High frequency in vitro plantlet regeneration in Solanum trilobatum L., an important ethno-medicinal plant and confirmation of genetic fidelity of R1 plantlets by using ISSR and RAPD markers. Vegetos 32(4):508–520
Phan NM, Hong TDT, Le Thanh TN, Trong TN, Quoc LN, Trong DT, Quan HN, Bui LCH, Diep XKN, Trong DB, Dinh TM, Tan PN (2021) Hepatoprotection and phytochemistry of the Vietnamese herbs Cleome chelidonii and Cleome viscosa stems. J Chem 19:2021
Pittampalli B, Jogam P, Thampu RK, Abbagani S, Peddaboina V (2022) High-frequency plant regeneration and genetic homogeneity assessment of regenerants by molecular markers in turmeric (Curcuma longa L.). In Vitro Cell Dev Biol-Plant 58(1):169–180
Rashmi R, Trivedi MP (2014) Effect of various growth hormone concentration and combination on callus induction, nature of callus and callogenic response of Nerium odorum. Appl Biochem Biotechnol 172(5):2562–2570
Rathore NS, Rathore N, Shekhawat NS (2013) In vitro propagation and micromorphological studies of Cleome gynandra: a C4 model plant closely related to Arabidopsis thaliana. Acta Phys Plan 35(9):2691–2698
Reddy CS, Raju VS (2001) A new variety of Cleome chelidonii L. f. (Cleomaceae). J Econ Taxon Bot 25(1):217–218
Rizza A, Walia A, Lanquar V, Frommer WB, Jones AM (2017) In vivo gibberellin gradients visualized in rapidly elongating tissues. Nat Plants 3(10):803–813
Rohela GK, Shabnam AA, Shukla P, Aurade R, Gani M, Yelugu S, Sharma SP (2018a) In vitro clonal propagation of PPR-1, a superior temperate mulberry variety. Ind J Biotech 17(4):619–625
Rohela GK, Jogam P, Shabnam AA, Shukla P, Abbagani S, Ghosh MK (2018b) In vitro regeneration and assessment of genetic fidelity of acclimated plantlets by using ISSR markers in PPR-1 (Morus sp.): an economically important plant. Sci Horti 241:313–321
Rohela GK, Jogam P, Mir MY, Shabnam AA, Shukla P, Abbagani S, Kamili AN (2020) Indirect regeneration and genetic fidelity analysis of acclimated plantlets through SCoT and ISSR markers in Morus alba L. cv. Chinese white. Biotechnol Rep 25:e00417
Rohela GK, Jogam P, Saini P, Sandhya D, Peddaboina V, Shekhawat MS (2022) Assessing the genetic stability of in vitro raised plants. In: Gupta S, Chaturvedi P (eds) Commercial scale tissue culture for horticulture and plantation crops. Springer, Singapore, pp 245–276
Sandhya D, Jogam P, Manokari M, Shekhawat MS, Jadaun JS, Allini VR, Abbagani S (2021) High-frequency in vitro propagation and assessment of genetic uniformity and micro-morphological characterization of Origanum majorana L.—a highly traded aromatic herb. Biocatal Agric Biotechnol 34:102024
Sandhya D, Jogam P, Venkatapuram AK, Savitikadi P, Peddaboina V, Allini VR, Abbagani S (2022) Highly efficient Agrobacterium-mediated transformation and plant regeneration system for genome engineering in tomato. Saudi J Biol Sci 29(6):103292
Savitikadi P, Jogam P, Rohela GK, Ellendula R, Sandhya D, Allini VR, Abbagani S (2020) Direct regeneration and genetic fidelity analysis of regenerated plants of Andrographis echioides (L.)—an important medicinal plant. Ind Crops Prod 155:112766
Shyamkumar B, Anjaneyulu C, Giri CC (2003) Multiple shoot induction from cotyledonary node explants of Terminalia chebula. Biol Plant 47(4):585–588
Simoes C, Albarello N, Callado CH, Castro TCD, Mansur E (2010) Somatic embryogenesis and plant regeneration from callus cultures of Cleome rosea Vahl. Braz Arch Biol Technol 53(3):679–686
Sirangi S, Jogam P, Nemali G, Ajmeera R, Abbagani S, Raju VS (2020) Intraspecific genetic variation in Corynandra chelidonii (Angiosperms: Cleomaceae) as revealed by SCoT, ISSR and RAPD analyses. J Plant Biotechnol 47(4):289–297
Sirangi S, Jogam P, Rohela GK, Ragan A, Raju VS (2021) Micropropagation of endemic Corynandra chelidonii var. pallae (Cleomaceae) through nodal explants and validation of their genetic integrity by ISSR markers. Vegetos 35(2):511–519
Suarez Padron IE, Perez Meza PM, Lopez Diaz CM (2020) Evaluation of sucrose and GA3 in an in vitro shoot culture of Alpinia purpurata (Zingiberaceae). Ciencia y Tecnología Agropecuaria 21(2):1–12
Sumitha V, Gurulakshmi M (2015) Antioxidant and free radical scavenging activity of leaf extracts of Cleome chelidonii Linn. Int J Innov Pharma Biosci Res Technol 2:228–236
Thomas TD, Maseena EA (2006) Callus induction and plant regeneration in Cardiospermum halicacabum Linn. an important medicinal plant. Sci Hortic 108(3):332–336
Vemula S, Koppula T, Jogam P, Mohammed M (2020) In vitro high frequency multiplication and assessment of genetic fidelity of Corallocarpus epigaeus: an endangered medicinal plant. Vegetos 33(1):63–73
Venkatachalam P, Jayabalan N (1997) Effect of auxins and cytokinins on efficient plant regeneration and multiple-shoot formation from cotyledons and cotyledonary-node expiants of groundnut (Arachis hypogaea L.) by in vitro culture technology. Appl Biochem Biotechnol 67(3):237–247
Zhang M, Tucker GC (2008) Cleomaceae. In: Wu CY, Raven PH, Hong DY (eds) Flora of China, vol 7. Science Press, Beijing, pp 518–521
Author Information
Plant Systematics Laboratory, Department of Botany, Kakatiya University, Warangal, India