Isoelectric precipitation of protein from pea pod and evaluation of its physicochemical and functional properties
Pooja B. K., Sethi Shruti, Bhardwaj Rakesh, Chawla Gautam, Kumar Rajesh, Joshi Alka, Bhowmik Arpan
Research Articles | Published: 30 June, 2023
First Page: 1131
Last Page: 1141
Views: 3214
Keywords: Freeze drying, FTIR, Iso-electric precipitation, Pea pod waste, Protein extraction
Abstract
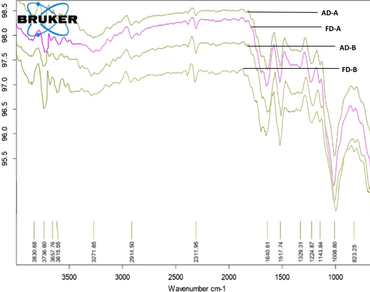
Pea pod shell as a solid waste in processing industries remains unutilized and is discarded as waste. Pea pod powder (PPP) prepared from pea pod waste was found to have 15.79% protein on dry weight basis (db) making it an excellent source of sustainable and vegan protein. In the present study, protein was extracted from PPP using two protocols (A and B) and subjected to air drying (AD-A and AD-B of method A and B, respectively) and freeze drying (FD-A and FD-B of method A and B, respectively). Based on the protein content, the best pH combination for protein extraction and precipitation was identified by employing factorial analysis of variance. Results revealed that pH combination 12.0 (alkali extraction)—3.5 (protein precipitation) was optimum for maximum protein yield (AD-A 48.68%, FD-A 50.64%, AD-B 60.45% and FD-B 63.10% db) from pea pod powder. FTIR spectra for all samples showed characteristic peaks corresponding to the secondary structure of proteins. Further, upon functionality evaluation FD-B protein was found best with higher protein content, high foaming capacity, emulsion stability, low moisture with good foam stability and emulsion capacity. However, sample AD-B was competitive with FD-B in terms of protein yield (60.33% vs 62.76% db), functionality and better handling. Therefore, we recommend heat assisted extraction method of pea pod protein that has been dried under forced air as a feasible low-cost alternative for manufacturing protein concentrate. Thus, the obtained pea pod protein can be incorporated as a high value supplement in human diets.
References
Abdel ESM, Shehata AA, El-Mahdy AR, Youssef MM (1986) Extractability and functional properties of some legume proteins isolated by three different methods. J Sci Food Agric 37:553–559. https://doi.org/10.1002/jsfa.2740370608
Akyuz S, Akyuz T, Celik O, Atak C (2018) FTIR spectroscopy of protein isolates of salt-tolerant soybean mutants. J Appl Spectrosc 84:1019–1023. https://doi.org/10.1007/s10812-018-0580-1
Altschul AM, Wilcke HL (1974) New protein foods. Academic Press, Orlando 5:20–23
Anson ML, Pader M (1957) U. S. Patent No. 2,785,155; U.S. Patent and Trademark Office: Washington, DC
AOAC (2000) Association of Official Analytical Chemists. Official methods of analysis, (17th ed). Association of Official Analytical Chemists (AOAC), Gaithersburg
Arise AK, Nwachukwu ID, Aluko RE, Amonsou EO (2017) Structure, composition and functional properties of storage proteins extracted from bambara groundnut (Vigna subterranea) landraces. Int J Food Sci Technol 52:1211–1220. https://doi.org/10.1111/ijfs.13386
Arogundade LA, Tshay M, Shumey D, Manazie S (2006) Effect of ionic strength and/or pH on extractability and physic-functional characterization of broad bean (Vicia faba L.) protein concentrate. Food Hydrocoll 20:1124–1134. https://doi.org/10.1016/j.foodhyd.2005.12.010
Azizi AF, Sethi S, Joshi A, Singh AM, Raigond P, Singh MK, Yadav RK (2020) Biochemical and functional attributes of raw and boiled potato flesh and peel powders for suitability in food applications. J Food Sci Technol 57:3955–3965. https://doi.org/10.1007/s13197-020-04424-3
Belghith-Fendri L, Chaari F, Kallel F, Zouari-Ellouzi S, Ghorbel R, Besbes S, Ellouz-Chaabouni S, Ghribi-Aydi D (2016) Pea and broad bean pods as a natural source of dietary fiber: The impact on texture and sensory properties of cake. J Food Sci 81:C2360–C2366. https://doi.org/10.1111/1750-3841.13448
Boye J, Zare F, Pletch A (2010) Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res Int 43:414–431. https://doi.org/10.1016/j.foodres.2009.09.003
Carbonaro M, Nucara A (2009) Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 38:679–690. https://doi.org/10.1007/s00726-009-0274-3
Carr RL (1965) Evaluating flow properties of solids. Chem Eng J72:163–168
Daba SD, Morris CF (2021) Pea proteins: Variation, composition, genetics, and functional properties. Cereal Chem 99:8–20. https://doi.org/10.1002/cche.10439
Damodaran S, Parkin KL, Fennema OR (2007) Fennema’s Food Chemistry. CRC press, pp 266–269.
Deng J, Sun T, Cao W, Fan D, Cheng N, Wang B, Gao H, Yang H (2014) Extraction optimization and functional properties of proteins from kiwi fruit (Actinidia chinensis Planch.) seeds. Int J Food Prop 17:1612–1625. https://doi.org/10.1080/10942912.2013.772197
Du M, Xie J, Gong B, Xu X, Tang W, Li X, Li C, Xie M (2018) Extraction, physicochemical characteristics and functional properties of mung bean protein. Food Hydrocoll 76:131–140. https://doi.org/10.1016/j.foodhyd.2017.01.003
Dumas JBA (1831) Procédés De L’analyseorganique. Ann Chim Phys 47:198–205
Eke OS, Akobundu ENT (1993) Functional properties of yam bean (Sphenu stenocarpa) seed flour as affected by processing. Food Chem 48:337–340. https://doi.org/10.1016/0308-8146(93)90314-6
Eneche HE, Owheruo OJ (2005) Proximate composition and functional properties of African yam bean/maize flour blends for various food preparation. In: Proceeding of 29th Annual Conference/AGM, NIFST, 179–180.
Eromosele CO, Arogundade LA, Eromosele IC, Ademuyiwa O (2008) Extractability of African yam bean (Sphenostylis stenocarpa) protein in acid, salt and alkaline aqueous media. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2007.11.003
Fang L, Xiang H, Sun-Waterhouse D, Cui C, Lin J (2020) Enhancing the usability of pea protein isolate in food applications through modifying its structural and sensory properties via deamidation by glutaminase. J Agric Food Chem 68:1691–1697. https://doi.org/10.1021/acs.jafc.9b06046
Fellows P (2005) Food Processing Technology: Principles and Practice. CRC Press, Washington, DC, Freezing
Feyzi S, Varidi M, Zare F, Varidi MJ (2015) Fenugreek (Trigonella foenumgraecum) seed protein isolate: Extraction optimization, amino acid composition, thermo and functional properties. J Sci Food Agric 95:3165–3176. https://doi.org/10.1002/jsfa.7056
Feyzi S, Milani E, Golimovahhed QA (2017) Grass pea (Lathyrus sativus L.) protein isolate: Study of extraction optimization, protein characterizations, structure and functional properties. Food Hydrocoll 74:187–196. https://doi.org/10.1016/j.foodhyd.2017.07.031
Garg D, Chakraborty S, Gokhale JS (2020) Optimizing the extraction of protein from Prosopis cineraria seeds using response surface methodology and characterization of seed protein concentrate. LWT Food Sci Technol. https://doi.org/10.1016/j.lwt.2019.108630
Hall GM (1996) Methods of testing protein functionality. Blackie Academic and Professional, London, UK, p 265
Hanan E, Rudra SG, Sagar VR, Sharma V (2020) Utilization of pea pod powder for formulation of instant pea soup powder. J Food Process Preserv. https://doi.org/10.1111/jfpp.14888
Hausner HH (1967) Friction conditions in a mass of metal powder. Int J Powder Metall 3:7–13
Joshi M, Adhikari B, Aldred P, Panozzo JF, Kasapis S (2011) Physicochemical and functional properties of lentil protein isolates prepared by different drying methods. Food Chem 129:1513–1522. https://doi.org/10.1016/j.foodchem.2011.05.131
Kinsella JE (1976) Functional properties of soy proteins. J Am Oil Chem Soc 56:242–258. https://doi.org/10.1007/BF02671468
Lam CY, Can Karaca A, Tyler RT, Nickerson MT (2018) Pea protein isolates: Structure, extraction, and functionality. Food Rev Int 34:126–147. https://doi.org/10.1080/87559129.2016.1242135
Mateos-Aparicio I, Redondo-Cuenca A, Villanueva-Suárez M (2012) Broad bean and pea by products as sources of fibre-rich ingredients: Potential antioxidant activity measured in vitro. J Sci Food Agric 92:697–703. https://doi.org/10.1002/jsfa.4663
Mondor M, Hernández-Álvarez AJ (2022) Processing technologies to produce plant protein concentrates and isolates. In: Manickavasagan A, Lim LT, Ali A (ed) Plant Protein Foods, Springer, Cham, pp 61–108. https://doi.org/10.1007/978-3-030-91206-2_3
Mwasaru MA, Muhammad K, Bakar J, Che-Man YB (1999) Effects of isolation technique and conditions on the extractability, physicochemical and functional properties of pigeonpea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolates. Food Chem 67:435–443. https://doi.org/10.1016/S0308-8146(99)00150-8
Neto VQ, Narain N, Silva JB, Bora PS (2001) Functional properties of raw and heat processed cashew nut (Anarcardium occidentale L.) kernel protein isolate. Foods 45:258–262. https://doi.org/10.1002/1521-3803(20010801)45
Perović MN, Jugović ZDK, Antov MG (2020) Improved recovery of protein from soy grit by enzyme-assisted alkaline extraction. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2019.109894
Pooja BK, Sethi S, Bhardwaj R, Joshi A, Bhowmik A, Grover M (2022) Investigation of physicochemical quality and textural attributes of muffins incorporated with pea pod powder as a source of dietary fiber and protein. J Food Process Preserv. https://doi.org/10.1111/jfpp.16884
Qiaoyun CUI, Xinghong NI, Liang ZENG, Zheng TU, Jin LI, Kang SUN, Xinghui LI (2017) Optimization of protein extraction and decoloration conditions for tea residues. Hortic Plant J. https://doi.org/10.1016/j.hpj.2017.06.003
Ranganna S (2007) Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Publishing Co, Limited, New Delhi
Sethi S, Joshi A, Seth K, Bhardwaj R, Yadav A, Grover M (2022) Phytonutrient content, antioxidant potential and acceptability of muffins functionalized with soy and citrus industry waste. J Food Process Preserv. https://doi.org/10.1111/jfpp.16606
Shen Y, Tang X, Li Y (2020) Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127823
Singh P, Kumar SN, Bawa AS (2008) Functional and edible uses of soy proteins products. Compr Rev Food Sci Food Saf 7:14–28. https://doi.org/10.1111/j.1541-4337.2007.00025.x
Tripathi K, Gore PG, Pandey A, Bhardwaj R, Singh N, Chawla G, Kumar A (2019) Seed morphology, quality traits and imbibition behaviour study of atypical lentil (Lens culinaris Medik.) from Rajasthan. India Genet Resour Crop Evol 66:697–706. https://doi.org/10.1007/s10722-019-00745-1
Ullao JA, Rosas-Ulloa P, Ulloa-Rangel BE (2011) Physicochemical and functional properties of a protein isolate produced from safflower (Carthamus tinctorius L.) meal by ultrafiltration. J Sci Food Agric 91:572–577. https://doi.org/10.1002/jsfa.4227
Upasana VD (2018) Nutritional evaluation of pea peel and pea peel extracted byproducts. Int J Food Sci Nutr 3:65–67
Wu H, Wang Q, Tiezheng M, Ren J (2009) Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Res Int 42:343–348. https://doi.org/10.1016/j.foodres.2008.12.006
Zhong C, Wang R, Zhou Z, Jia SR, Tan ZL, Han PP (2012) Functional properties of protein isolates from Caragana koshinskii Kom. extracted by three different methods. J Agric Food Chem 60:10337–10342. https://doi.org/10.1021/jf303442u
Author Information
Division of Food Science and Postharvest Technology, ICAR-Indian Agricultural Research Institute, New Delhi, India