In vitro shoot formation and enrooted mini-corm production by direct organogenesis in saffron (crocus sativus L.)
Research Articles | Published: 25 May, 2023
First Page: 1045
Last Page: 1050
Views: 2181
Keywords: Saffron, Regeneration, Bud sprouting, Adventitious shoots, Progeny corms
Abstract
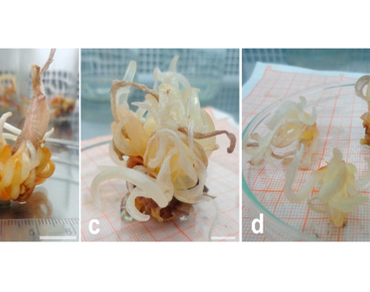
Saffron’s triploidy and male sterility result in generative limitations that highly impact its regeneration. Therefore, its natural reproduction is exclusively vegetative through progeny corms. The present work aims to improve in vitro saffron bud sprouting and adventitious shoot regeneration through direct organogenesis using different combinations of plant growth regulators, and then investigate novel conditions for mini-corm production. Murashige and Skoog medium (MS) containing 1 mg/L of 6-benzyl aminopurine (BAP) and α-naphthalene acetic acid (NAA) resulted in the highest bud sprouting rate (96.67%) and growth length (8.87 cm ± 0.27 cm). The best adventitious shoot initiation rate of 80% with 10.2 ± 0.23 shoots per explant was obtained using 0.5 mg/L NAA and 2.75 mg/L BAP. Remarkably, the critical step of mini-corm regeneration was improved using ½ MS, 6% sucrose, 1 mg/L NAA, and dark incubation. The corms produced weighed 7.9 ± 0.8 g, and 93.80% developed roots with an average of 14.9 ± 3.1 roots per corm. Improving the critical stages of saffron tissue culture is essential to meet the urgent need for its large-scale and low-cost regeneration.
References
Agayev YMoghlu, Fernandez JA, Zarifi E (2009) Clonal selection of saffron (Crocus sativus L.): the first optimistic experimental results. Euphytica 169. https://doi.org/10.1007/s10681-009-9946-z
Ahmad M, Zaffar G, Mir SD et al (2011) Saffron (Crocus sativus L.) strategies for enhancing productivity. Res J Med Plant 5. https://doi.org/10.3923/rjmp.2011.630.649
Benjlil H, Elkassemi K, Aït Hamza M et al (2020) Plant-parasitic nematodes parasitizing saffron in Morocco: structuring drivers and biological risk identification. Appl Soil Ecol 147:103362. https://doi.org/10.1016/j.apsoil.2019.103362
Chib S, Thangaraj A, Kaul S et al (2020) Development of a system for efficient callus production, somatic embryogenesis and gene editing using CRISPR/Cas9 in Saffron (Crocus sativus L). Plant Methods 16. https://doi.org/10.1186/s13007-020-00589-2
Devi K, Sharma M, Ahuja PS (2014) Direct somatic embryogenesis with high frequency plantlet regeneration and successive cormlet production in saffron (Crocus sativus L). South Afr J Bot 93. https://doi.org/10.1016/j.sajb.2014.04.006
Devi K, Sharma M, Singh M, Singh Ahuja P (2011) In vitro cormlet production and growth evaluation under greenhouse conditions in saffron (Crocus sativus L.) - a commercially important crop. Eng Life Sci 11. https://doi.org/10.1002/elsc.201000080
Díaz-Vivancos P, Majourhat K, Fernández JA et al (2011) Study of the antioxidant enzymatic system during shoot development from cultured intercalar meristems of saffron. Plant Growth Regul 65. https://doi.org/10.1007/s10725-011-9581-2
Freytag C, Pabar SA, Demeter Z et al (2017) Production and characterization of tissue cultures of four Crocus species from the carpathian basin. Acta Biol Cracoviensia Ser Bot 59. https://doi.org/10.1515/abcsb-2017-0009
Hosseini A, Razavi BM, Hosseinzadeh H (2018) Pharmacokinetic Properties of Saffron and its active components. Eur. J. Drug Metab. Pharmacokinet. 43
Majourhat K, Fernández JA, Martínez-Gómez P, Piqueras A (2007) Enhanced plantlet regeneration from cultured meristems in sprouting buds of saffron corms. In: Acta Horticulturae
Mir I, Wajida NA S, et al (2014) In vitro development and regeneration of microcorms in saffron (Crocus sativus L). Afr J Biotechnol 13. https://doi.org/10.5897/ajb2013.12831
Mir JI, Ahmed N, Wani SH et al (2010) In vitro development of microcorms and stigma like structures in saffron (Crocus sativus L). Physiol Mol Biol Plants 16. https://doi.org/10.1007/s12298-010-0044-4
Mykhailenko O, Kovalyov V, Goryacha O et al (2019) Biologically active compounds and pharmacological activities of species of the genus Crocus: a review. Phytochemistry 162
Nemati Z, Blattner FR, Kerndorff H et al (2018) Phylogeny of the saffron-crocus species group, Crocus series Crocus. Mol Phylogenet Evol 127. https://doi.org/10.1016/j.ympev.2018.06.036
Parray JA, Kamili AN, Hamid R, Husaini AM (2012) In vitro cormlet production of saffron (Crocus sativus L. Kashmirianus) and their flowering response under greenhouse. GM Crops Food 3. https://doi.org/10.4161/gmcr.21365
Renau-Morata B, Moyá L, Nebauer SG et al (2013) The use of corms produced under storage at low temperatures as a source of explants for the in vitro propagation of saffron reduces contamination levels and increases multiplication rates. Ind Crops Prod 46. https://doi.org/10.1016/j.indcrop.2013.01.013
Rubio-Moraga A, Ahrazem O, Pérez-Clemente RM et al (2014) Apical dominance in saffron and the involvement of the branching enzymes CCD7 and CCD8 in the control of bud sprouting. BMC Plant Biol 14. https://doi.org/10.1186/1471-2229-14-171
Sharma KD, Rathour R, Sharma R et al (2008) In vitro cormlet development in Crocus sativus. Biol Plant 52. https://doi.org/10.1007/s10535-008-0136-y
Shokrpour M (2019) Saffron (Crocus sativus L.) breeding: Opportunities and challenges. Advances in plant breeding strategies. Industrial and Food Crops
Yasmin S, Nehvi FA (2013) Seasonal bud sprouting: a challenge for in vitro micropropagation in saffron. Vegetos 26. https://doi.org/10.5958/j.2229-4473.26.2.068.
Author Information
Department of Environment and Life Sciences, Faculty of Applied Sciences, Ibn Zohr University, Agadir, Morocco