HR-LCMS Profiling of phytochemical constituents and evaluation of antioxidant, antibacterial, anti-cancerous and anti-inflammatory potentials, plasma biocompatibility and cytotoxicity of Grewia orbiculata Rottler
Research Articles | Published: 02 January, 2023
First Page: 1446
Last Page: 1457
Views: 3608
Keywords: n Grewia orbiculatan , Antibacterial activity, Antioxidant property, Anti-cancerous activity, Catechin, Gallic acid, Quercetin
Abstract
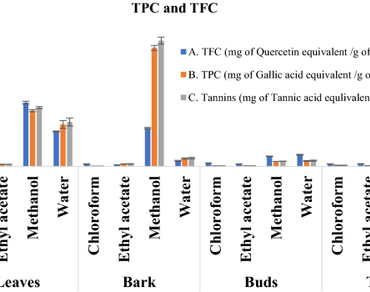
Infectious diseases are one of the main reasons that are causing a greater number of deaths in the world owing to their strong resistance development and evolution. There is an immediate urgency for the discovery of drugs with a new class or new mode of action to combat these resistant bugs. In the past few decades, we have not been able to find new antibiotics, which are effective on resistant bugs. Instead of searching for synthetic molecules, if we divert our search for alternative sources that are abundant in nature, we can easily find new molecules. Plants are the best as they are known to possess complex molecules that are strong in their potency while being relatively safe for the host and tough on pathogens. With this rationale, the study was conducted to assess the phytochemical constituents of different parts of plant Grewia orbiculata Rottler using different solvents and to elucidate the biological activities. From qualitative analysis of all extracts, Methanolic Extract of Bark (MEB) and Ethyl acetate Extract of Leaf (EEL) were found to be rich in total phenolics and total flavonoids. Major phytochemicals found in MEB were Catechin, Epicatechin, and Carnitine and in EEL were Quinin acid, Gallic acid, Catechol, Isoquinoline, Coumaric acid, Kaempferol, and Quercetin of G. orbiculata. Upon testing the biopotentials of these extracts, it was found that among the different solvent extracts of leaves, twigs, buds, and bark, MEB showed the highest biological potential and therapeutic value. The antioxidant property of MEB assessed through DPPH and ABTS assays resulted in an IC50 value of 50 µg/mL and 36 µg/mL, respectively. The metal chelating property of MEB gave a FRAP value of 24 ± 0.093 mmol/g equivalent to that of Tannic acid. Further, MEB was found to possess very good antibacterial activity against human pathogens such as Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus faecalis, Enterococcus faecium, Streptococcus epidermidis, and Mycobacterium smegmatis. In addition, MEB also showed good anti-cancerous property against A549 cells, having IC50 value of 98.73 µg/mL. The anti-inflammatory assay with MEB showed protection of BSA denaturation up to a concentration of 1000 µg/mL. Finally, the biocompatibility assay with blood showed no significant agglutination of RBCs up to a concentration of 200 µg/mL and cytotoxicity of MEB resulted in less than 50% inhibition of HTE cell proliferation at the highest concentration of 320 µg/mL, proving its non-toxic nature towards normal cells. Our study is the first to report and evaluate the therapeutic value of the plant G. orbiculata. MEB was found to possess very good therapeutic potential and can be used as potent antimicrobial agent to treat deadly human infections.
References
Aadesariya MK, Gauni BM, Duggirala SM, Ram VR, Vyas SJ (2017a) Antibacterial activity of Abutilon pannosum and Grewia tenax leaves extracts. World J Pharm Res 6:1259–1274
Aadesariya MK, Ram VR, Dave PN (2017b) Extraction, isolation, and identification of useful phyto constituents from dichloromethane leave extract of Abutilon Pannosum and Grewia Tenax using Q-TOF LC/MS. IJARCS 4(10):1–14
Afsar T, Razak S, Khan MR, Mawash S, Almajwal A, Shabir M, Haq IU (2016) Evaluation of antioxidant, anti-hemolytic and anticancer activity of various solvent extracts of Acacia hydaspica R Parker aerial parts. BMC Complement Altern 16:258
Altundag EM, Gencalp D, Ozbilenler C, Toprak K, Kerküklü N (2020) In vitro antioxidant, anti-inflammatory, and anti-cancer activities of methanolic extract of Asparagus horridus grows in north Cyprus. Turk J Biochem 45(4):365–372
Berdy J (2012) Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot 65(8):385–395
Borges A, Ferreira C, Saavedra MJ, Simões M (2013) Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist 19:256–265
Chatatikun M, Chiabchalard A (2013) Phytochemical screening and free radical scavenging activities of orange baby carrot and carrot (Daucus carota Linn.) root crude extracts. J Chem Pharm Res 5(4):97–102
Cinti S, Malani A, Riddell J (2008) Infectious diseases. Clinical Men’s Health. Elsevier, pp 182–206
De Torre MP, Cavero RY, Calvo MI, Vizmanos JL (2019) A simple and a reliable method to quantify antioxidant activity in vivo. Antioxidants 8(5):142
Fair RJ, Tor Y (2014) Antibiotics, and bacterial resistance in the 21st century. Perspect Med Chem 6:25–64
Gopal J, Muthu M, Paul D, Kim DH, Chun S (2016) Bactericidal activity of green tea extracts: the importance of catechin containing nano particles. Sci Rep. https://doi.org/10.1038/srep19710
Goyal PK (2012) Phytochemical and pharmacological properties of the genus Grewia: a review. Int J Pharm Pharm Sci 4(4):72–78
Greenwell M, Rahman PK (2015) Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res 6(10):4103–4112
Harborne JB (1984) Phytochemical methods. A guide to modern technique of plant analysis. Chapman & Hall, Springer Netherlands, pp 78–210
Herald T, Gadgil P (2012) Tilley M (2012) High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J Sci Food Agric 92(11):2326–2331
Khatua S, Ghosh S, Acharya K (2017) Simplified methods for microtiter-based analysis of in vitro antioxidant activity. Asian J Pharm 11(2):S327–S335
Koc A, Ozkan T, Karabay AZ, Sunguroglu A, Aktan F (2011) Effect of L-carnitine on the synthesis of nitric oxide in RAW 264·7 murine macrophage cell line. Cell Biochem Funct 29(8):679–685
Kumar S, Singh B, Bajpai V (2022) Traditional uses, phytochemistry, quality control and biological activities of genus Grewia. Phytomedicine plus 2(3):100290
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC (2005) Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 339(1):69–72
Murray C et al (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399(10325):629–655
Nordin N, Yeap SK, Rahman HS (2019) In vitro cytotoxicity, and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci Rep 9:1614
Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Nur H, Ismail AF, Sharif S, RamaKrishna S, Berto F (2020a) Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 9(12):1309
Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Nur H, Ismail AF, Sharif S, RamaKrishna S, Berto F (2020b) Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 9:1309
Qamar M, Akhtar S, Barnard R, Sestili P, Ziora Z, Lazarte C, Ismail T (2022) Anti-inflammatory and anticancer properties of Grewia Asiatica crude extracts and fractions: a bioassay-guided approach. Biomed Res Int 2022:1–14
Rajavel T, Mohankumar R, Archunan G et al (2017) Beta sitosterol and Daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci Rep 7:3418
Selvam NT, Vengatakrishnan V, Murugesan S, Kumar SD (2010) Antioxidant and antiproliferative actvitiy of methanolic extract of Grewia tiliaefolia (Vahl) bark in different cancer cell lines. IJPLS 1(2):54–60
Shariati A, Moradabadi A, Azimi T, Ghaznavi-Rad E (2020) Wound healing properties and antimicrobial activity of platelet-derived biomaterials. Sci Rep 10:1032
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics. CA Cancer J Clin 72(1):7–33
Sofowora A (1993) Medicinal plants, and traditional medicine in Africa. Spectrum Books Ltd., Ibadan, pp 191–289
Suguna M, Umesha S (2022a) Taxonomical review on Grewia orbiculata Rottl., an Indian ethno-medicinal plant. J Med Plants Stud 10(4):194–196
Suguna M, Umesha S (2022b) Phytochemical composition, pharmacological properties, and therapeutic activities of genus: Grewia. J Pharmacogn Phytochem 11(4):263–272
Trease GE, Evans WC (1989) Pharmacognosy, 11th edn. Bailliere Tindall, London, pp 45–50
Waliullah GU, Rauf A, Siddiqui BS, Rehman TU, Qaisar MN, Pakistan K (2011) Chemical Constituents and biological screening of Grewia optiva drummond ex. Burret Whole Plant AEJAES 11:542–546
Yang D, Wang T, Long M, Li P (2020) Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev 2020:1–13
Author Information
Department of Biotechnology, University of Mysore, Mysuru, India