Detection of chemical compounds by GC–MS and evaluation of antioxidant, anti-inflammatory, antibacterial and anti-cancer potential in methanolic extracts of Corallocarpus epigaeus (Rottler) Hook. f.
*Article not assigned to an issue yet
Research Articles | Published: 17 December, 2025
First Page: 0
Last Page: 0
Views: 53
Keywords: n Corallocarpus epigaeusn , Gas chromatography-mass spectroscopy, Methanolic extracts, Phytoconstituents, Bioactive compounds
Abstract
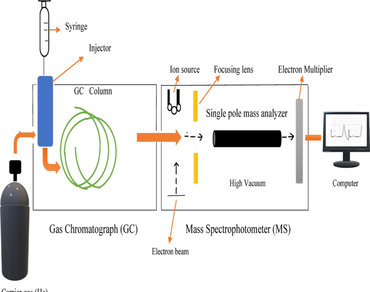
The Gas Chromatography-Mass Spectroscopy (GC–MS) technique was used in the present work to identify bioactive compounds found in various plant parts of Corallocarpus epigaeus (C. epigaeus). Corallocarpus epigaeus is rich in many phytochemicals such as phenols, steroids, flavonoids, alkaloids, and tannins. The samples were rinsed, dried, and pulverized into a powder. The powdered leaf, stem, and root were combined with methanol and incubated for 48 h using cold maceration technique. Chromatograms and bioactive parameters of methanolic extracts from the leaves, stem and roots of C. epigaeus were obtained using the GC–MS method. According to chromatogram analysis, 8 compounds in leaf methanolic extracts; stem methanolic extracts 14 and root methanolic extracts 20 bioactive compounds were identified. Among the above, these are key bioactive compounds Lupeol, Phytol, Glycocholic acid, n- Hexadecanoic acid, 9-octadecenoic acid methyl ester, Stigmasterol, B-sitosterol, Diethyl Phthalate, Dimethyl sulfoxided, Allomatrine, Hexadecanoic acid methyl ester, 9,12-Octadecadienoic acid (Z, Z), methyl ester, Melezitose, 3-O-Methyl-d-glucose, n-Hexadecanoic acid derivatives are synthesized from leaf, stem and root explants of C. epigaeus. These designated chemicals have biological properties that may combat the incurable human illnesses.
References
Abubakar MN, Majinda RR (2016) GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines (Basel) 3(1):3. https://doi.org/10.3390/medicines3010003
Afzal M, Shahid M, Jamil A (2014) Phytochemical spectrum of essential oil of Paganum harmala by GC-MS and antimicrobial activity using sequential solvents fractions and essential oil. Asian J Chem 26(2):574–578
Ali H, Dixit S, Ali D, Alqahtani SM, Alkahtani S, Alarifi S (2015) Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug des Devel Ther 9:2793. https://doi.org/10.2147/DDDT.S83514
Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M (2012) Anti‐inflammatory property of n‐hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug des 80(3):434–439. https://doi.org/10.1111/j.1747-0285.2012.01418.x
Ashwathanarayana R, Naika RA (2017) Study on antioxidant and cytotoxic properties of Olea dioica Roxb. crude extract and its pure compound collected from Western Ghats, Karnataka, India. Asian J Pharm Clin Res 10(2):356–367. https://doi.org/10.22159/ajpcr.2017.v10i2.15727
Awuchi CG (2019) Medicinal plants: the medical, food, and nutritional biochemistry and uses. Int J Adv Acad Res 5(11):220–241
Belakhdar G, Benjouad A, Abdennebi EH (2015) Determination of some bioactive chemical constituents from Thesium humile Vahl. J Mater Environ Sci 6(10):2778–2783
Capriotti K, Capriotti JA (2012) Dimethyl sulfoxide: history, chemistry, and clinical utility in dermatology. J Clin Aesthet Dermatol 5(9):24
Chelliah R, Ramakrishnan S, Antony U (2017) Nutritional quality of Moringa oleifera for its bioactivity and antibacterial properties. Int Food Res J 24(2):825
Choi JM, Lee EO, Lee HJ, Kim KH, Ahn KS, Shim BS, Kim NI, Song MC, Baek NI, Kim SH (2007) Identification of campesterol from Chrysanthemum coronariumL. and its antiangiogenic activities. Phytother Res 21(10):954–959. https://doi.org/10.1002/ptr.2189
Dias MK, Madusanka DM, Han EJ, Kim MJ, Jeon YJ, Kim HS, Fernando IP, Ahn G (2020) (−)-loliolide isolated from Sargassum horneri protects against fine dust-induced oxidative stress in human keratinocytes. Antioxidants 9(6):474. https://doi.org/10.3390/antiox9060474
Fluxome WC (2007) Analysis using GC-MS. Microb Cell Fact 6:1–7
Gallo MB, Sarachine MJ (2009) Biological activities of lupeol. Int J Biomed Pharm Sci 3(1):46–66
Gnananath K, Reddy KR, Gudur PK, Bheemanapally K, Karka SR, Avvari SK (2013) Evaluation of antidiabetic activity in Corallocarpus epigaeus rhizomes. Int Curr Pharm J 2(3):53–56
Hema R, Kumaravel S, Alagusundaram K (2011) GC/MS determination of bioactive components of Murraya koenigii. J Am Sci 7(1):80–83
Hino T, Takabe M, Suzuki-Migishima R, Yokoyama M (2007) Cryoprotective effects of various saccharides on crypreserved mouse sperm from various strains. Reprod Med Biol 6(4):229–233
Hirotani H, OhigashiH KM, Koshimizu K, Takahashi E (1991) Inactivation of T5 phage by cis-vaccenic acid, an antivirus substance from Rhodopseudomonas capsulata, and by unsaturated fatty acids and related alcohols. FEMS Microbiol Lett 77(1):13–17. https://doi.org/10.1111/j.1574-6968.1991.tb04314.x
Hussein HJ, Hadi MY, Hameed IH (2016) Study of chemical composition of Foeniculum vulgare using Fourier transform infrared spectrophotometer and gas chromatography-mass spectrometry. J Pharmacognosy Phytother 8(3):60–89
Ikele CB, Obiezue RN, Okoye IC, Otuu CA (2016) Lipid peroxidation and some antioxidant enzymes of C. gariepinus fingerlings exposed to diethyl phthalate. J Adv Biol Biotechnol. https://doi.org/10.9734/JABB/2016/20137
Ishnava KB, Konar PS (2020) In vitro anthelmintic activity and phytochemical characterization of Corallocarpus epigaeus (Rottler) Hook. f. tuber from ethyl acetate extracts. Bull Natl Res Cent 44(1):1–0
Ishnava K, Kotadia R, Patel S (2015) Nutritional properties and chemical composition of Corallocarpus epigaeus (Arn.) Cl: as remedy to control diabetes mellitus. Chiang Mai J Sci 42(4):806–815
Islam MT, Ali ES, Uddin SJ, Shaw S, Islam MA, Ahmed MI, Shill MC, Karmakar UK, Yarla NS, Khan IN, Billah MM (2018) Phytol: a review of biomedical activities. Food Chem Toxicol 121:82–94. https://doi.org/10.1016/j.fct.2018.08.032
Jay TM, DienelGA CNF, Mori K, Nelson T, Sokoloff L (1990) Metabolic stability of 3-O-methyl-D-glucose in brain and other tissues. J Neurochem 55(3):989–1000. https://doi.org/10.1111/j.1471-4159.1990.tb04588.x
Jayawardena TU, Kim HS, Sanjeewa KA, Kim SY, Rho JR, Jee Y, Ahn G, Jeon YJ (2019) Sargassum horneri and isolated 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one (HTT); LPS-induced inflammation attenuation via suppressing NF-κB, MAPK and oxidative stress through Nrf2/HO-1 pathways in RAW 264.7 macrophages. Algal Res 40:101513. https://doi.org/10.1016/j.algal.2019.101513
Jeong JB, Hong SC, Jeong HJ, Koo JS (2011) Anti-inflammatory effect of 2-methoxy-4-vinylphenol via the suppression of NF-κB and MAPK activation, and acetylation of histone H3. Arch Pharm Res 34(12):2109–2116. https://doi.org/10.1007/s12272-011-1214-9
Kadhim MJ, Al-Rubaye AF, Hameed IH (2017) Determination of bioactive compounds of methanolic extract of Vitis vinifera using GC-MS. Int J Toxicol Pharmacol Res 9(2):113–126
Kumar PP, Kumaravel S, Lalitha C (2010) Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biochem Res 4(7):191–195
Fadipe La, Haruna AK, Mohammed I (2014) Antibacterial activity of 1, 2-benzenedicarboxylic acid, dioctyl ester isolated from the ethyl acetate soluble sub-portion of the unripe fruits of Nauclea latifolia 2 (1): 223–230
Lan W, Lin S, Li X, Zhang Q, Qin W (2017) Chemical composition of the leaf and stem essential oil of Adenophorae radix. AIP Conf Proc 1820:030001. https://doi.org/10.1063/1.4977258
Li MC (2015) Effect and mechanism of allomatrine in proliferation and invasion in vitro inhibition of human lung cancer A549 cell line. Chin Pharm J 24:1111–1116
Lo YL, Ho CT, Tsai FL (2008) Inhibit multidrug resistance and induce apoptosis by using glycocholic acid and epirubicin. Eur J Pharm Sci 35(1–2):52–67. https://doi.org/10.1016/j.ejps.2008.06.003
Mahesh R, Babu VS, Narayana MS, Naik DN, Malothu R (2012) Hepatoprotective activity of leaves of Corallocarpus epigaeus in carbon tetrachloride induced rats. Ramesh Malothu. et al. Int J Biol Pharm Res 3(4):567–570
Moustafa MF, Alamri SA, Taha TH, Alrumman SA (2013) In vitro antifungal activity of Argemone ochroleuca sweet latex against some pathogenic fungi. Afr J Biotechnol. https://doi.org/10.5897/AJB12.2649
Palchykov VA, Zazharskyi VV, Brygadyrenko VV, Davydenko PO, Kulishenko OM, Borovik IV (2020) Chemical composition and antibacterial effect of ethanolic extract of Buxus sempervirens on cryogenic strains of microorganisms in vitro. Chem Data Collect 25:100323. https://doi.org/10.1016/j.cdc.2019.100323
Pejin B, Ciric A, Glamoclija J, Nikolic M, Sokovic M (2015) In vitro anti-quorum sensing activity of phytol. Nat Prod Res 29(4):374–377. https://doi.org/10.1080/14786419.2014.945088
Pohlit AM, Lopes NP, Gama RA, Tadei WP, de Andrade Neto VF (2011) Patent literature on mosquito repellent inventions which contain plant essential oils: a review. Planta Med 77(06):598–617. https://doi.org/10.1055/s-0030-1270723
Ravi L, Krishnan K (2017) Research article cytotoxic potential of N-hexadecanoic acid extracted from Kigelia pinnata leaves. Asian J Cell Biol 12:20–27. https://doi.org/10.3923/ajcb.2017.20.27
Saleem M (2009) Lupeola novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett 285(2):109–115. https://doi.org/10.1016/j.canlet.2009.04.033
Semwal P, Painuli S, Badoni H, Bacheti RK (2018) Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin Phytosci 4(1):1–6. https://doi.org/10.1186/s40816-018-0090-y
Thangavel A, Jayaseelan M, Thangaraj N (2014) Evaluation of antioxidant and anti-inflammatory activities of Corallocarpus epigaeus (Hook. f.) rhizomes. Int J Res Pharm Biomed Sci 5(1):18–24
Vasantha K, Priyavardhini S, Tresina SP, Mohan VR (2012) Antifungal activity of Corrallocarpus epigaeus (Hook. f.) against cancer. Biosci Discov 3(1):87–90
Veera S, Chirumamilla P, Taduri S (2020) High efficiency in vitro regeneration and genetic stability of Corallocarpus epigaeus-an endangered medicinal plant. Plant Tissue Cult Biotechnol 30(2):219–229
Veera S, Chirumamilla P, Dharavath SB, Maduru N, Taduri S (2023) Facile green synthesis of silver nanoparticles using Corallocarpus epigaeus leaf extract: structural, photoluminescence and antibacterial properties. Chem Data Collect 1(45):101032. https://doi.org/10.1016/j.cdc.2023.101032
Author Information
Department of Biotechnology, Kakatiya University, Warangal, India