Comparative analysis of desiccation tolerance in Oeosporangium elegans and Mickelopteris cordata
Rudresh Hosahalli Somasundara, Divyashree Thippesh, Yathisha Neeragunda Shivaraj, Dwarakanath Venkatesha, Kumar Hulikall Shivashankara Santhosh, Sharathchandra Ramasandra Govind
Research Articles | Published: 10 July, 2023
First Page: 1244
Last Page: 1256
Views: 3625
Keywords: Desiccation, Pteridophytes, Relative water content, Anti-oxidant enzymes
Abstract
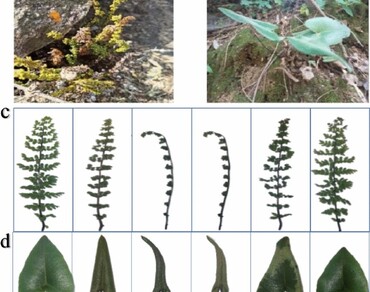
Vegetative desiccation tolerance of Oeosporangium elegans and Mickelopteris cordata was evaluated in the current study to understand physiological responses towards desiccation and rehydration in different pteridophyte species. Desiccation tolerance (DT) was measured at different stages of water loss [Water Loss-1 (WL1), Water Loss-2 (WL2), Desiccated (D)] and gain [Water Gain-1 (WG1) and Rehydrated (R)]. Our results shown that relative water content (RWC) and photosynthetic pigments were reduced but not completely destroyed at desiccation. Also, the CO2 exchange rate and Fv/Fm ratio were significantly lower during desiccation. However, concentrations of anthocyanin, proline, lipid peroxidation, superoxide radicals, and activity of antioxidant enzymes were found to be significantly higher at all the time points tested during desiccation. Our results shown that non-Selaginella species of pteridophytes also have the innate ability to counter physiological desiccation through various adaptation methods.
References
Abouzari A, Fakheri BA (2015) Reactive oxygen species: generation, oxidative damage, and signal transduction. Int J Life Sci 9(5):3–17. https://doi.org/10.3126/ijls.v9i5.12699
Alejo-Jacuinde G, Herrera-Estrella L (2022) Exploring the high variability of vegetative desiccation tolerance in pteridophytes. Plants (basel, Switzerland) 11(9):1222. https://doi.org/10.3390/plants11091222
Alici E, Arabaci G (2016) Determination of SOD, POD, PPO and CAT enzyme activities in Rumex obtusifolius L. Annu Res Rev Biol 11(3):1–7. https://doi.org/10.9734/ARRB/2016/29809
Anwar Hossain M, Hoque MA, Burritt DJ, Fujita M (2014) Proline protects plants against abiotic oxidative stress. In: Oxidative damage to plants. Elsevier, pp 477–522. https://doi.org/10.1016/B978-0-12-799963-0.00016-2
Banupriya TG, Ramyashree C, Akash D, Yathisha NS, Sharathchandra RG (2020) A study on mechanisms of response and adaptation to desiccation tolerance in Selaginella wightii, a pteridophyte species of semiarid region in south India. Int J Bot Stud 5(5):357–364. http://www.botanyjournals.com/archives/2020/vol5/issue5/5-5-108
Bartels D (2005) Desiccation tolerance studied in the resurrection plant Craterostigma plantagineum. Integr Comp Biol 45(5):696–701. https://doi.org/10.1093/icb/45.5.696
Chaitra H, Lavanya PM, Pavitra K, Sharathchandra RG, Kambalagere Y, Yathisa NS (2017) Identification and Evaluation of Cellular and Biochemical Changes in Responses to Desiccation Tolerance in Cheilanthes Albomarginata Fern. IOSR J Biotechnol Biochem 03:25–32. https://doi.org/10.9790/264X-03042532
Deeba F, Pandey V, Pathre U, Kanojiya S (2009) Proteome analysis of detached fronds from a resurrection plant Selaginella bryopteris—response to dehydration and rehydration. J Proteom Bioinform 02(02):108–116. https://doi.org/10.4172/jpb.1000067
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149(1):78–90. https://doi.org/10.1007/BF00386231
Farrant JM (2000) A comparison of mechanisms of desiccation tolerance among three angiosperm resurrection plant species. Plant Ecol 151(1):29–39. https://doi.org/10.1023/A:1026534305831
Gaff DF, Oliver M (2013) The evolution of desiccation tolerance in angiosperm plants: a rare yet common phenomenon. Funct Plant Biol 40(4):315. https://doi.org/10.1071/FP12321
Gechev TS, Benina M, Obata T, Tohge T, Sujeeth N, Minkov I, Hille J, Temanni M-R, Marriott AS, Bergström E, Thomas-Oates J, Antonio C, Mueller-Roeber B, Schippers JHM, Fernie AR, Toneva V (2013) Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cell Mol Life Sci CMLS 70(4):689–709. https://doi.org/10.1007/s00018-012-1155-6
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta (BBA) Gen Subj 990(1):87–92. https://doi.org/10.1016/S0304-4165(89)80016-9
Gururani MA, Venkatesh J, Ganesan M, Strasser RJ, Han Y, Kim J-I, Lee H-Y, Song P-S (2015) In vivo assessment of cold tolerance through chlorophyll-a fluorescence in transgenic Zoysia grass expressing mutant phytochrome A. PLoS ONE 10(5):e0127200. https://doi.org/10.1371/journal.pone.0127200
Harten JB, Eickmeier WG (1987) Comparative desiccation tolerance of three desert pteridophytes: response to long-term desiccation. Am Midl Nat 118(2):337–347. https://doi.org/10.2307/2425790
Kranner I, Beckett R, Hochman A, Nash TH (2008) Desiccation-tolerance in lichens: a review. Bryologist 111(4):576–593
Lee J, Durst RW, Wrolstad RE, Eisele T, Giusti MM, Hach J, Hofsommer H, Koswig S, Krueger DA, Kupina S, Martin SK, Martinsen BK, Miller TC, Paquette F, Ryabkova A, Skrede G, Trenn U, Wightman JD (2005) Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 88(5):1269–1278. https://doi.org/10.1093/jaoac/88.5.1269
Maehly AC (2006) The assay of catalases and peroxidases. In: Methods of biochemical analysis. Wiley, New York, pp 357–424. https://doi.org/10.1002/9780470110171.ch14
Neeragunda Shivaraj Y, Barbara P, Gugi B, Vicré-Gibouin M, Driouich A, Ramasandra Govind S, Devaraja A, Kambalagere Y (2018) Perspectives on structural, physiological, cellular, and molecular responses to desiccation in resurrection plants. Scientifica 2018:1–18. https://doi.org/10.1155/2018/9464592
Neeragunda Shivaraj Y, Plancot B, Ramdani Y, Gügi B, Kambalagere Y, Jogaiah S, Driouich A, Ramasandra Govind S (2021) Physiological and biochemical responses involved in vegetative desiccation tolerance of resurrection plant Selaginella brachystachya. 3 Biotech 11(3):135. https://doi.org/10.1007/s13205-021-02667-1
Oliver MJ, Farrant JM, Hilhorst HWM, Mundree S, Williams B, Bewley JD (2020) Desiccation tolerance: avoiding cellular damage during drying and rehydration. Annu Rev Plant Biol 71(1):435–460. https://doi.org/10.1146/annurev-arplant-071219-105542
Pampurova S, Van Dijck P (2014) The desiccation tolerant secrets of Selaginella lepidophylla: what we have learned so far? Plant Physiol Biochem 80:285–290. https://doi.org/10.1016/j.plaphy.2014.04.015
Pandey V, Ranjan S, Deeba F, Pandey AK, Singh R, Shirke PA, Pathre UV (2010) Desiccation-induced physiological and biochemical changes in resurrection plant, Selaginella bryopteris. J Plant Physiol 167(16):1351–1359. https://doi.org/10.1016/j.jplph.2010.05.001
Porembski S (2011) Evolution, diversity, and habitats of poikilohydrous vascular plants. In: Lüttge U, Beck E, Bartels D (eds) Plant desiccation tolerance. Springer, pp 139–156. https://doi.org/10.1007/978-3-642-19106-0_8
Proctor M (2009) Desiccation tolerance in some British ferns. Fern Gazette 18:216–234
Proctor MCF, Smirnoff N (2000) Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: chlorophyll fluorescence and inhibitor experiments. J Exp Bot 51(351):1695–1704. https://doi.org/10.1093/jexbot/51.351.1695
Quintanilla LG, Aranda I, Clemente-Moreno MJ, Pons-Perpinyà J, Gago J (2023) Ecophysiological differentiation among two resurrection ferns and their allopolyploid derivative. Plants 12(7):Article 7. https://doi.org/10.3390/plants12071529
Sankara Rao K, Swamy RK, Deepak K, Singh RA, Gopalakrishna Bhat K (2019a). Flora of Peninsular India. http://peninsula.ces.iisc.ac.in/plants.php?name=Mickelopteriscordata
Sankara Rao K, Swamy RK, Deepak K, Singh RA, Gopalakrishna Bhat K (2019b). Flora of Peninsular India. http://peninsula.ces.iisc.ac.in/plants.php?name=Oeosporangiumelegans
Sosa K (2022) Potential desiccation tolerance shown in Cheilanthes ecuadorensis (Pteridaceae). Am Fern J 112(1):73–77. https://doi.org/10.1640/0002-8444-112.1.73
Vander Willigen C, Pammenter NW, Mundree S, Farrant J (2001) Some physiological comparisons between the resurrection grass, Eragrostis nindensis, and the related desiccation-sensitive species E. curvula. Plant Growth Regul 35(2):121–129. https://doi.org/10.1023/A:1014425619913
Veljovic-Jovanovic S (2006) Senescence- and drought-related changes in peroxidase and superoxide dismutase isoforms in leaves of Ramonda serbica. J Exp Bot 57(8):1759–1768. https://doi.org/10.1093/jxb/erl007
Yathisha NS, Barbara P, Gügi B, Yogendra K, Jogaiah S, Azeddine D, Sharatchandra RG (2020) Vegetative desiccation tolerance in Eragrostiella brachyphylla: biochemical and physiological responses. Heliyon 6(9):e04948. https://doi.org/10.1016/j.heliyon.2020.e04948
Yobi A, Wone BWM, Xu W, Alexander DC, Guo L, Ryals JA, Oliver MJ, Cushman JC (2012) Comparative metabolic profiling between desiccation-sensitive and desiccation-tolerant species of Selaginella reveals insights into the resurrection trait: comparative metabolomics of desiccation tolerance. Plant J 72(6):983–999. https://doi.org/10.1111/tpj.12008
Author Information
Department of Studies and Research in Biotechnology, Tumkur University, Tumakuru, India