Assessment of total phenolics, flavonoids, and antioxidant properties within the genus Cotoneaster in Kashmir Himalayas
Research Articles | Published: 27 October, 2023
First Page: 2514
Last Page: 2522
Views: 3195
Keywords: Methanolic extracts, Polyphenols, n Cotoneastern , DPPH, Natural antioxidants
Abstract
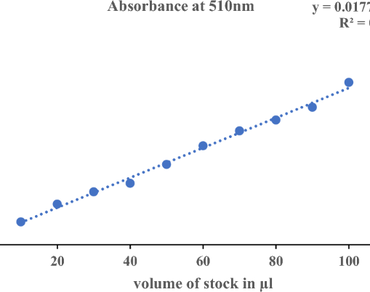
Cotoneaster, a member of the Rosaceae family comprises around 300 species globally and has attained a significant place in traditional medicine. Although polyphenols are known to be prevalent in most Cotoneaster species, very little is known about their specific content and antioxidant activity. Therefore, the current study aimed to evaluate the content of total phenolics, total flavonoids, and antioxidant activity of six Cotoneaster species viz, Cotoneaster integerrimus, Cotoneaster affinis, Cotoneaster nummularia, Cotoneaster horizontalis, Cotoneaster rosea, and Cotoneaster microphylla growing in Kashmir Himalaya. The folin-Ciocalteu method and aluminium chloride method were used to evaluate the total phenolics and total flavonoids respectively. Also, the antioxidant properties of the methanolic extracts were assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Reducing power assays. The results revealed noteworthy antioxidant activity and total phenolic content. The fruit extracts of C. rosea and C. nummularia had the highest content of phenolic and flavonoids (297.05 0.63 f mg/g GAE and 30.47 0.22 d mg/g QE, respectively). The highest antioxidant activity observed by the DPPH was found in the fruit extract of C. rosea (IC50 = 3.45 ± 0.01). Moreover, Antioxidant activity and total phenolic content were shown to be significantly correlated. This study underscores the potential therapeutic value of these Cotoneaster species and positions them as a valuable source of potent natural antioxidants for pharmaceutical and nutraceutical applications. However, further research is required to evaluate the toxicity and other biological properties of these crude extracts, along with the identification of different compounds present within them.
References
Bag GC, Devi PG, Bhaigyabati TH (2015) Assessment of Total Flavonoid Content and Antioxidant Activity of Methanolic Rhizome extract of three Hedychium species of Manipur valley. Int J Pharm Sci Rev Res 30(1):154–159
Bartish IV, Hylmö B, Nybom H (2001) RAPD analysis of interspecific relationships in presumably apomictic Cotoneaster species. Euphytica 120:273–280
Cakilcioglu U, Turkoglu I (2010) An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey). J Ethnopharmacol 132(1):165–175
Chanda S, Dave R (2009) In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr J Microbiol Res 3(13): 981–996
Chu YH, Chang CL, Hsu HF (2000) Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric 80(5):561–566. https://doi.org/10.1002/(SICI)1097-0010(200004)80:5%3c561:AID-JSFA574%3e3.0.CO;2
Dorman HJD, Hiltunen R (2004) Fe (III) reductive and free radical-scavenging properties of summer savory (Satureja hortensis L.) extract and subfractions. Food Chem 88(2):193–199. https://doi.org/10.1016/j.foodchem.2003.12.039
Doshi P, Adsule P, Banerjee K (2006) Phenolic composition and antioxidant activity in grapevine parts and berries (Vitis vinifera L.) cv. Kishmish Chornyi (Shared Seedless) during maturation. Int J Food Sci Technol 41(SUPPL. 1):1–9. https://doi.org/10.1111/j.1365-2621.2006.01214.x
Fakhri M, Azadbakht M, Yousefi SS, Mousavinasab SN, Farhadi R, Azadbakht M (2016) Medicinal plants for treatment of neonatal jaundice by community of attars (traditional healers) of several urban areas in Mazandaran province, northern of Iran. Br J Med Med Res 1(11):1–3
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress, and the biology of ageing. Nature 408(6809):239–247
Haq F, Ahmad H, Alam M (2011) Traditional uses of medicinal plants of Nandiar Khuwarr catchment (District Battagram), Pakistan. J. Med. Plant Res 5(1):39–48
IBM Spss Statistics (2015) IBM, SPSS Statistics for windows (23). IBM Corp, Armonk
Kahl R, Kappus H (1993) Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Zeitschrift Für Lebensmittel-Untersuchung Und Forschung 196:329–338
Kicel A, Kolodziejczyk-Czepas J, Owczarek A, Marchelak A, Sopinska M, Ciszewski P, Nowak P, Olszewska MA (2018) Polyphenol-rich extracts from Cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules. https://doi.org/10.3390/molecules23102472
Krzemińska B, Dybowski MP, Klimek K, Typek R, Miazga-Karska M, dos Santos Szewczyk K (2022) The anti-acne potential and chemical composition of two cultivated Cotoneaster Species. Cells. https://doi.org/10.3390/cells11030367
Liu Z, Yang, (2018) Antisolvent precipitation for the preparation of high polymeric procyanidin nanoparticles under ultrasonication and evaluation of their antioxidant activity in vitro. Ultrason Sonochem 43:208–218. https://doi.org/10.1016/j.ultsonch.2018.01.019
Swati S, Manjula R, Kattupalli S, Vennela Y, Tanuja K (2018) A phyto pharmacological review on Cotoneaster microphyllus species. J Pharm Sci Res 10(9):2166–2168
Mansouri A, Embarek G, Kokkalou E, Kefalas P (2005) Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem 89(3):411–420. https://doi.org/10.1016/j.foodchem.2004.02.051
Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, Leitão SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15(2):127–130. https://doi.org/10.1002/ptr.687
Mititelu RR, Pădureanu R, Băcănoiu M, Pădureanu V, Docea AO, Calina D, Barbulescu AL, Buga AM (2020) Inflammatory and oxidative stress markers-mirror tools in rheumatoid arthritis. Biomedicines. https://doi.org/10.3390/BIOMEDICINES8050125
Mohamed SA, Sokkar NM, El-Gindi O, Ali ZY, Alfishawy IA (2012) Phytoconstituents investigation, anti-diabetic and anti-dyslipidemic activities of Cotoneaster horizontalis. Life Sci J 9(2):394–403
Moure A, Cruz JM, Franco D, Domı́nguez JM, Sineiro J, Domı́nguez H, Núñez MJ, Parajó JC (2001) Natural antioxidants from residual sources. Food Chem 72(2):145–171
Olszewska MA, Nowak S, Michel P, Banaszczak P, Kicel A (2010) Assessment of the content of phenolics and antioxidant action of inflorescences and leaves of selected species from the genus sorbus sensu stricto. Molecules 15(12):8769–8783. https://doi.org/10.3390/molecules15128769
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 1(2):270–278
Popoviciu DR, Negreanu-Pirjol T, Motelica L, Stefan B, Pirjol N (2020) Carotenoids, Flavonoids, Total Phenolic Compounds and Antioxidant Activity of Two Creeping Cotoneaster Species Fruits Extracts. Rev. Chim, 71(3): 136–142. https://doi.org/10.37358/Rev
R Core Team (2022) R: a language and environment for statistical computing (4.1.2). R Foundation for Statistical Computing Vienna, Austria. https://www.r-project.org/. Accessed 5 Jan 2023
Salehi B, Sharifi-Rad J, Cappellini F, Reiner Z, Zorzan D, Imran M, Sener B, Kilic M, El-Shazly M, Fahmy NM, Al-Sayed E, Martorell M, Tonelli C, Petroni K, Docea AO, Calina D, Maroyi A (2020) The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Front Pharmacol 11:1300. https://doi.org/10.3389/fphar.2020.01300
Sarkar C, Chaudhary P, Jamaddar S, Janmeda P, Mondal M, Mubarak MS, Islam MT (2022) Redox activity of flavonoids: impact on human health, therapeutics, and chemical safety. Chem Res Toxicol 35(2):140–162. https://doi.org/10.1021/ACS.CHEMRESTOX.1C00348
Schöneich C (1999) Reactive oxygen species and biological aging: a mechanistic approach. Exp Gerontol 34(1):19–34
Shah SM, Hussain F (2012) Ethnomedicinal plant wealth of Mastuj valley, Hindukush range, district Chitral, Pakistan. J Med Plant Res 6(26):4328–4337
Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I, Prakash Mishra A (2020) Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front Physiol 11:694
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 3:144–158
Sravani T, Paarakh P (2011) Evaluation of anthelmintic activity of Hedychium spicatum Buch. Int J Res Pharm Sci 2(1):66–68
Teo WL (2021) Diagnostic and management considerations for “maskne” in the era of COVID-19. J Am Acad Dermato 84(2):520–521. https://doi.org/10.1016/j.jaad.2020.09.063
Wojdyło A, Oszmiański J, Czemerys R (2007) Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 105(3):940–949. https://doi.org/10.1016/j.foodchem.2007.04.038
Zengin G, Uysal A, Gunes E, Aktumsek A (2014) Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): a potential source for functional food ingredients and drug formulations. PLoS ONE 9(11):e113527
Author Information
Cytogenetics and Plant Molecular Biology Research Laboratory, Department of Botany, University of Kashmir, Srinagar, India