Antimicrobial potential of in vitro and in vivo plant parts of Paeonia emodi Wallich Ex Royle (Himalayan peony)
Research Articles | Published: 18 June, 2022
First Page: 494
Last Page: 505
Views: 3531
Keywords: Paeonia emodi , Callus, Minimum inhibitory concentration (MIC), Solvent, Altitude
Abstract
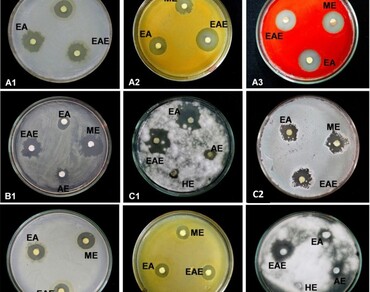
The antimicrobial potential of in vitro (callus) and in vivo (leaf and rhizome) plant parts of Himalayan peaony (Paeonia emodi) has been investigated with particular reference to solvent polarity and altitudinal variation (1827–2350 m above sea level). Five different solvents, i.e., methanol, ethanol, acetone, ethyl acetate, and hexane, and three groups of microorganisms (bacteria, actinobacteria, and fungi) were considered to detect antimicrobial potential. The qualitative estimations were done using the agar well diffusion method, while quantitative analysis was based on the dilution method. The plant parts showed significant activities against all three groups of microorganisms in qualitative bioassays. For example, ethanolic extract of leaf collected from Triyuginarayan showed the highest antibacterial activity (19.27 ± 0.23 mm) with Serratia marcescens. Maximum inhibition (13.28 ± 0.12 mm) of actinobacteria was observed in the methanolic extracts of leaf collected from Gwaldum. Methanolic extract of leaf collected from Pootiwasa showed higher antifungal activity (21.10 ± 0.06 mm). In vitro callus extract also showed activity against bacteria, actinobacteria, and Fusarrium sp. Minimum inhibitory concentrations ranged from 100 to 900 µg/mL. Pearson correlation analysis revealed that solvent polarity exhibited a significantly positive relationship with antimicrobial activity in both plant parts (leaf and rhizome).The increasing altitude negatively affected the antimicrobial activity in P. emodi plant parts. The antimicrobial activity of leaf and rhizome of P.emodi varied with the solvent types and their growing conditions. The study will have implications for developing some antifungal and antimicrobial agents, which can be used to prepare different pharmaceuticals and health products from P. emodi.
References
Abraham J, Thomas TD (2012) Antibacterial activity of medicinal plant Cyclea peltata (Lam) Hooks & Thoms. Asian Pac J Trop Dis 2:S280–S284
Adhikari P, Pandey A (2017) Taxus wallichiana Zucc (Himalayan Yew) in antimicrobial perspective. Adv Biotechnol Microbiol 4:555–650
Adhikari P, Pandey A, Agnihotri V, Pandey V (2018) Selection of solvent and extraction method for determination of antimicrobial potential of Taxus wallichiana Zucc. Res Pharm 8:1–9
Ahmad M, Malik K, Tariq A, Zhang G, Yaseen G, Rashid N, Sultana S, Zafar M, Ullah K, Khan MPZ (2018) Botany, ethnomedicines, phytochemistry and pharmacology of Himalayan paeony (Paeonia emodi Royle). J Ethnopharmacol 220:197–219
Ali H, Sannai J, Sher H, Rashid A (2011) Ethnobotanical profile of some plant resources in Malam Jabba valley of Swat. Pak J Med Plant Res 5(18):4676–4687
Baskaran C, Bai VR, Velu S, Kumaran K (2012) The efficacy of Carica papaya leaf extract on some bacterial and a fungal strain by well diffusion method. Asian Pac J Trop Dis 2:S658–S662
Chávez de Paz LE, Bergenholtz G, Svensäter G (2010) The effects of antimicrobials on endodontic biofilm bacteria. J Endod 36(1):70–77
CLSI (2008) Reference method for broth dilution antifungal susceptibilitytesting of yeasts, 3rd edn. M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA
Felhi S, Baccouch N, Ben SH, Smaoui S, Allouche N, Gharsallah N, Kadri A (2016) Nutritional constituents, phytochemical profiles, in vitro antioxidant and antimicrobial properties, and gas chromatography-mass spectrometry analysis of various solvent extracts from grape seeds. Food Sci Biotechnol 25:1537–1544
Felhi S, Daoud A, Hajlaoui H, Mnafgui K, Gharsallah N, Kadri A (2017) Solvent extraction effects on phytochemical constituents profiles, antioxidant and antimicrobial activities and functional group analysis of Ecballium elaterium seeds and peels fruits. Food Sci Technol 37:483–492
Jacobs MR (2001) Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin Microbiol Infect 7(11):589–596
Johnson D, Booth RE, Whiteley AS, Bailey MJ, Read DJ, Grime JP, Leake JR (2003) Plant community composition affects the biomass, activity and diversity of microorganisms in limestone grassland soil. Eur J Soil Sci 54(4):671–678
Jugran AK, Chaudhary WY, Bahukhandi A, Bhatt ID, Rawal RS, Dhyani PP (2016) Effect of processing and storage methods on the nutritional, anti-nutritional, and antioxidant properties of Paeonia emodi. Wall Ex Royle Appl Biochem Biotechnol 180:322–337
Kallel F, Driss D, Chaari F, Belghith L, Bouaziz F, Ghorbel R, Chaabouni SE (2014) Garlic (Allium sativum L.) husk waste as a potential source of phenolic compounds: influence of extracting solvents on its antimicrobial and antioxidant properties. Ind Crops Prod 62:34–41
Khameneh B, Iranshahy M, Soheili V, Bazzaz BSF (2019) Review on plants antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control 8:118
Kitzberger CSG, Smaˆnia A Jr, Pedrosa RC, Frreira SRS (2007) Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J Food Eng 80:631–638
Mandal SD, Passari AK, Ghatak S, Mishra VK, Senthil Kumar N, Singh BP (2015) Total phenol content, antioxidant and antimicrobial capability of traditional medicinal plants of Mizoram, Eastern Himalayas, Northeast India. EC Agric 2:350–357
Metrouh-Amira H, Duarteb CMM, Maiza F (2015) Solvent effect on total phenolic contents, antioxidant, and antibacterial activities of Matricaria pubescens. Ind Crops Prod 67:249–256
Mufti FUD, Ullah H, Bangash A, Khan N, Hussain S, Ullah F, Jamil M, Jabeen M (2012) Antimicrobial activities of Aerva javanica and Peonia emodi plants. Pak J Pharm Sci 25:565–569
Murashige T, Skoog FA (1962) revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Negi VS, Maikhuri RK (2016) Forest resources consumption pattern in Govind Wildlife Sanctuary, Western Himalaya. India J Environ Plan Manag 60(7):1235–1252
Pavarini DP, Pavarini SP, Niehues M, Lopes NP (2012) Exogenous influences on plant secondary metabolite levels. Anim Feed Sci Technol 176(1–4):5–16
Picerno P, Mencherini T, Sansone F, Gaudio PD, Granata I, Porto A, Aquino RP (2011) Screening of a polar extract of Paeonia rockii: composition and antioxidant and antifungal activities. J Ethnopharmacol 138:705–712
Prakash P, Joshi P, Purohit VK (2020) Impact on density of Paeonia emodi along altitudinal gradient in Garhwal Himalaya. India Appl Ecol Environ Sci 8(5):319–323
Rawat B, Gairola S, Bhatt A (2010) Habitat characteristics and ecological status of Paeonia emodi Wallich ex Royle: a high value medicinal plant of West Himalaya. Med Plants 2:121–125
Verma RS, Padalia RC, Chauhan A, Chanotiya CS (2015) Essential oil composition of Himalayan Peony (Paeonia emodi Royle). J Essent Oil Res 27:477–480
Yogabaanu U, Weber JFF, Convey P, Rizman-Idid M, Alias SA (2017) Antimicrobial properties and the influence of temperature on secondary metabolite production in cold environment soil fungi. Polar Sci 14:60–67
Author Information
G.B. Pant National Institute of Himalayan Environment, Almora, India