Antimicrobial activity and mycochemical profile of methanol extract from Pleurotus flabellatus
Pandey Aprajita Tiwari, Pandey Ishan, Kerkar Pranali, Singh M. P.
Research Articles | Published: 28 June, 2021
First Page: 619
Last Page: 629
Views: 3937
Keywords: Antimicrobial, Mycochemical, Pathogens, Extract, Methanol
Abstract
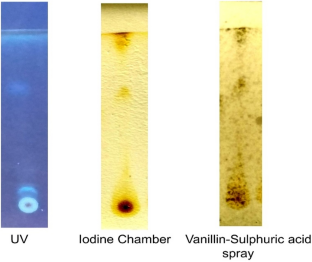
Pleurotus flabellatus is an edible mushroom possessing high nutritive as well as medicinal values. This study specifically investigated the antimicrobial properties of the methanol extract of P. flabellatus. Agar well diffusion and INT colorimetric assay methodologies were used to assess the antimicrobial activity of the extract against selected test pathogenic microorganisms viz. Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Proteus vulgaris NCIM 5266, Shigella flexeneri NCIM 5265, Proteus mirabilis NCIM 2300, Serratia marcescens NCIM 2078 and Moraxella catarrhalis NCIM 2795. Preliminary mycochemical screening revealed the presence of secondary metabolites such as flavonoids, diterpenoids, triterpenoids, alkaloids, phenols, carbohydrates, proteins, aminoacids, phlobatamins, terpenes and saponins, etc. which is responsible for antimicrobial activity. The thin-layer chromatography (TLC) eluted out 5 different compounds from the spot (extract) with solvent system chloroform + methanol (9.5:0.5) when visualized in UV, Iodine chamber and with sprayed acidified vanillin solution (0.1 g vanillin:28 ml methanol:1 ml sulphuric acid). The extract showed a mean zone of growth inhibition of 10.3 ± 0.3 to 17.6 ± 0.3 mm against the test pathogens. The methanol extracts showed bactericidal activity against all the test pathogens at MIC values of 26.03–83.33 mg/ml. Whereas the MBC values for methanol extract of P. flabellatus against all pathogens were recorded as 62.5–104.16 mg/ml. S. aureus which was resistant to commercial antibiotic penicillin gave highest antimicrobial activity (17.6 ± 0.3 mm) for methanol extract in comparison to other test pathogens. Serratia marcescens showed lowest antimicrobial activity with zone of inhibition 10.3 ± 0.3 mm. Among all the test pathogens, due to low MIC value of Proteus mirabilis, it showed higher total antibacterial Activity (TAA). Methanol extracts of P. flabellatus exhibited broad-spectrum of antimicrobial activity against test pathogens probably due to presence of several bioactive compounds that may serve as potential antimicrobial agents.
References
Abushaheen MA, Muzaheed Fatani AJ et al (2020) Antimicrobial resistance, mechanisms and its clinical significance. DIS-MON: DM. https://doi.org/10.1016/j.disamonth.2020.100971
Adebayo EA, Oloke JK, Ayandele AA et al (2012) Phytochemical, antioxidant and antimicrobial assay of mushroom metabolite from Pleurotuspulmonarius–LAU 09 (JF736658). J Microbiol Biotech Res 2:366–374. http://scholarsresearchlibrary.com/archive.html
Appiah T, Boakye YD, Agyare C (2017) Antimicrobial activities and time-kill kinetics of extracts of selected Ghanaian mushrooms. EvidBased Complement Alternat Med. https://doi.org/10.1155/2017/4534350
Bains A, Tripathi A (2016) Antibacterial activity and phytochemical screening of wild edible mushroom pleurotus ostreatuscollected from himachalpradesh. Int J Curr Adv Res 4:2320–5407. https://doi.org/10.21474/IJAR01
Barros L, Calhelha RC, Vaz JA et al (2007) Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur Food Res Technol 225:151–156. https://doi.org/10.1007/s00217-006-0394-x
Bele AA, Khale A, Archana M et al (2011) An overview on thin layer chromatography. Int J Pharm Sci Res 2:256–267 (www.ijpsr.com)
Bhardwaj A, Pal M, Srivastava M et al (2015) HPTLC based chemometrics of medicinal mushrooms J. Liq Chromatogr Relat Technol. https://doi.org/10.1080/10826076.2015.1050501
Bhardwaj K, Sharma A, Tejwan N (2020) Pleurotus macrofungi-assisted nanoparticle synthesis and its potential applications: a review. J Fungi. https://doi.org/10.3390/jof6040351
Boakye YD (2009) Anti-infective properties and time-kill kinetics of Phyllanthus muellerianus and its major constituent, Geraniin. Med Chem 6:95–104. https://doi.org/10.4172/2161-0444.1000332
Boucher HW, Talbot GH, Bradley JS et al (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. https://doi.org/10.1086/595011
Bubueanu C, Grigore A, Serban E et al (2017) HPTLC identification of bioactive compounds and antioxidant activity of Pleurotus ostreatus and Lentinusedodes extracts. Sci Bull Ser F Biotechnol XXI:343–348
Canli K, Altuner EM, Akata I (2015) Antimicrobial screening of Mniumstellare. Bangladesh J Pharmacol 10:321. https://doi.org/10.3329/bjp.v10i2.22463
Cateni F, Gargano ML, Procida G, Venturella G et al (2021) Mycochemicals in wild and cultivated mushrooms: nutrition and health. Phytochem Rev. https://doi.org/10.1007/s11101-021-09748-2
Chatterjee D, Halder D, Das S (2021) Varieties of mushrooms and their nutraceutical importance: a systematic review. JCDR. https://doi.org/10.7860/JCDR/2021/47240.14660
Durga SS, Eyini M, Rajendran J (2020) Mycochemical profiling of selected wild Basidiomycetes mushroom fungi of Alagar Hills, Madurai, Tamilnadu. Int J Adv Res Biol Sci. https://doi.org/10.22192/ijarbs.2020.07.10.005
Elisha IL, Botha FS, McGaw LJ et al (2017) The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complem Altern M 17:133. https://doi.org/10.1186/s12906-017-1645-z
Eloff JN (2004) Quantification the bioactivity of plant extracts during screening and bioassay guided fractionation. Phytomedicine 11:370–371
Fogarasi M, Diaconeasa ZM, Pop CR et al (2020) Elemental Composition, Antioxidant and Antibacterial Properties of Some Wild Edible Mushrooms from Romania. Agronomy. https://doi.org/10.3390/agronomy10121972
Gashaw G, Fassil A, Redi F (2020) Evaluation of the antibacterial activity of Pleurotus spp. cultivated on different agricultural wastes in Chiro, Ethiopia. Int J Microbiol. https://doi.org/10.1155/2020/9312489
Gebreyohannes G, Nyerere A, Bii C et al (2019) Investigation of antioxidant and antimicrobial activities of different extracts of Auricularia and Termitomyces species of mushrooms. Sci World J. https://doi.org/10.1155/2019/7357048
Giri S, Biswas G, Pradhan P et al (2012) Antimicrobial activities of basidiocarps of wild edible mushrooms of West Bengal, India. Int J Pharmtech Res 4:1554–1560
He F, Yang Y, Yang G et al (2010) Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control 21:1257–1262. https://doi.org/10.1016/j.foodcont.2010.02.013
Kalu A, Kenneth O (2017) Antimicrobial activity of Pleurotus squarrosulus on clinical pathogenic bacteria and fungi. Adv Microbiol 4:1–9. https://doi.org/10.9734/jamb/2017/34644
Kandasamy S, Chinnappan S, Thangaswamy S et al (2020) Assessment of antioxidant, antibacterial activities and bioactive compounds of the wild edible mushroom Pleurotussajor-caju. Int J Pept Res Ther 26:1575–1581. https://doi.org/10.1007/s10989-019-09969-2
Keepers TR, Gomez M, Celeri C et al (2014) Bactericidal activity, absence of serum effect, and time-kill kinetics of ceftazidime-avibactam against β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:5297–5305. https://doi.org/10.1128/AAC.02894-14
Khameneh B, Iranshahy M, Soheili V et al (2019) Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. https://doi.org/10.1186/s13756-019-0559-6
Landingin HRR, Francisco BE, Dulay RMR et al (2021) Mycochemical screening, proximate nutritive composition and radical scavenging activity of Cyclocybe cylindracea and Pleurotus cornucopiae. Curr Res Environ Appl Mycol 11:37–50. https://doi.org/10.5943/cream/11/1/3
Manandhar S, Luitel S, Dahal RK (2019) In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J Trop Med. https://doi.org/10.1155/2019/1895340
Mishra J, Bhardwaj A, Pal M (2018) High performance thin layer chromatography hyphenated with electrospray mass spectrometry for evaluation of nucleobases in two traditional Chinese medicinal mushrooms: a metabolomic approach. J Liq Chromatogr Relat Technol. https://doi.org/10.1080/10826076.2018.1539672
Mogasale VV, Saldanha P, Pai V et al (2021) A descriptive analysis of antimicrobial resistance patterns of WHO priority pathogens isolated in children from a tertiary care hospital in India. Sci Rep. https://doi.org/10.1038/s41598-021-84293-8
Mossebo DC, Metsebing B-P, Oba R et al (2020) Comparative evaluation of antifungal and antibacterial activities of crude extracts of Pleurotus sajor-caju, Pleurotus tuber-regium and Lentinus squarrosulus (Basidiomycota, Pleurotaceae, Lentinaceae) from Cameroon. EJBIO. https://doi.org/10.24018/ejbio.2020.1.5.97
Owusu E, Schwinger G, Dzomeku M et al (2017) Phytochemical, free radical scavenging activity and thin layer chromatography analysis of methanolic extracts of six wild mushroom species collected from the shai hills reserve of Ghana. Phcog J 9:16–22. https://doi.org/10.5530/pj.2017.6s.152
Oyetayo VO, Dong CH, Yao YJ (2009) Antioxidant and antimicrobial properties of aqueous extract from Dictyophoraindusiata. Open Mycol J 3:20–26. https://doi.org/10.2174/1874437000903010020
Özdal M, Gülmez Ö, Özdal G et al (2019) Antibacterial and antioxidant activity of mycelial extracts of different Pleurotus Species. Food Health 5:12–18. https://doi.org/10.3153/fh19002
Pandey AT, Pandey I, Hachenberger Y et al (2020a) Emerging paradigm against global antimicrobial resistance via bioprospecting of mushroom into novel nanotherapeutics development. Trends Food Sci Tech 106:333–344. https://doi.org/10.1016/j.tifs.2020.10.025
Pandey AT, Pandey I, Zamboni I et al (2020b) Traditional herbal remedies with a multifunctional therapeutic approach as an implication in COVID-19. Coatings 10:761. https://doi.org/10.3390/coatings10080761
Pandey AT, Pandey I, Kanase A et al (2021) Validating anti-infective activity of Pleurotus Opuntiae via standardization of its bioactive mycoconstituents through multimodal biochemical approach. Coatings. https://doi.org/10.3390/coatings11040484
Parihar S, Virani KD, Pithawala EA et al (2015) Phytochemical screening, total phenolic content, antibacterial and antioxidant activity of wild edible mushroom Pleurotus ostreatus. Int Res J Pharm 6:65–69. https://doi.org/10.7897/2230-8407.06115
Prakash KD, Sayali G (2017) Current quality control methods for standardization of herbal drugs. Int J Pharm 5:82–95
Prastiyanto ME, Rukmana RM, Saraswati DK et al (2020) Anticancer potential of methanolic extracts from Pleurotus species on raji cells and antibacterial activity against Methicillin-Resistant Staphylococcus aureus. Biodiversitas. https://doi.org/10.13057/biodiv/d211221
Rai M, Sen S, Acharya K (2013) Antimicrobial activity of four wild edible mushrooms from Darjeeling hills, West Bengal, India. Int J Pharmtech Res 5:949–956
Shree AKG, Balamurugan TSB, Manivasagan V et al (2016) Phytochemical, antioxidant and antitumor activity of edible mushroom Pleurotus ostreatus.Int J Adv Res Biol Sci 3:200–212. https://doi.org/10.22192/ijarbs
Singh AV, Jahnke T, Xiao Y et al (2019) Peptide-induced biomineralization of tin oxide (SnO2) nanoparticles for antibacterial applications. J Nanosci Nanotechnol. https://doi.org/10.1166/jnn.2019.16645
Singh AV, Kishore V, Santomauro G et al (2020) Mechanical coupling of puller and pusher active microswimmers influences motility. Langmuir. https://doi.org/10.1021/acs.langmuir.9b03665
Singh AV, Maharjan RS, Kanase A et al (2021) Machine-learning-based approach to decode the influence of nanomaterial properties on their interaction with cells. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.0c18470
Subbulakshmi M, Kannan M (2016) Cultivation and phytochemical analysis of wild mushrooms Daldiniaconcentrica and Pheolusschweinitzii from Tamilnadu, India. Eur J Exp Biol 6:46–54
Sudirman LI (2010) Partial purification of antimicrobial compounds isolated from mycelia of tropical lentinuscladopus LC4. Hayati 17:63–67. https://doi.org/10.4308/hjb.17.2.63
Swamydhas P, Lakshmanan G (2019) Culture of oyster mushroom, Pleurotus flabellatus on agro waste – paddy straw. Rev Res 8:1–10
Vamanu E (2012) In vitro antimicrobial and antioxidant activities of ethanolic extract of lyophilized mycelium of Pleurotus ostreatus PQMZ91109, 17. Molecules. https://doi.org/10.3390/molecules17043653
Waktola G, Temesgen T (2020) Pharmacological activities of Oyster mushroom (Pleurotus ostreatus). NRMJ. https://doi.org/10.21608/nrmj.2020.84017
Watkola G, Temesgen T (2018) Application of mushroom as food and medicine. AIBM. https://doi.org/10.19080/aibm.2018.11.555817
Yamaç M, Bilgili F (2006) Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharm Biol 44:660–667. https://doi.org/10.1080/13880200601006897
Author Information
Centre of Biotechnology, Nehru Science Complex, University of Allahabad, Prayagraj, India