Biotechnological exploitation of cyanobacterial photoprotective metabolites
Review Articles | Published: 16 February, 2022
Online ISSN : 2229-4473.
Website:www.vegetosindia.org
Pub Email: contact@vegetosindia.org
First Page: 281
Last Page: 297
Views: 1135
Keywords:
Cyanobacteria, Mycosporine-like amino acids, Scytonemin, Carotenoids, Sunscreens, Biotechnological activity
Abstract
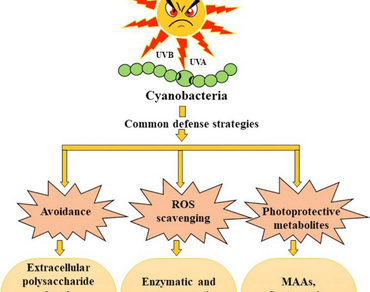
Cyanobacteria have received much attention in recent years due to their promising applications in the field of biotechnology and pharmaceutics. Ultraviolet radiation (UVR) has detrimental effects on the skin which has led to the commercial success of synthetic UV filters to diminish the deleterious effects of harmful highly energetic radiations. Cyanobacterial photoprotective metabolites (CPMs) such as mycosporine-like amino acids (MAAs), scytonemin and carotenoids increases skin s ability to retain water and because of this they are used in sunscreen products. Present day UV filters and synthetic moisturizing chemicals may also have disadvantageous effects on the skin. To overcome the devastating effects of UVR, CPMs are considered as natural photoprotectants and an alternative to the present day contrived UV filters. MAAs are considered to be a potential source of innovative bioactive metabolites that are highly fascinating from a biotechnological perspective showing multifarious biotechnological activities ranging from photoprotection to antioxidants, anti-inflammatory, anticancer, antiaging, immunomodulatory and visual venture. This review focuses on the gene cluster, biosynthetic pathway, protection against various stress and biotechnological exploitation of certain CPMs. These true multifunctional secondary metabolites have various important biotechnological applications and thus an attractive area for future research.
(*Only SPR Members can get full access. Click Here to Apply and get access)
References
Abed RMM, Garcia-Pichel F (2001) Long-term compositional changes after transplant in a microbial mat cyanobacterial community composition revealed using a polyphasic approach. Environ Microbiol 3:53–62. https://doi.org/10.1046/j.1462-2920.2001.00159
Abed RMM, Dobretsov S, Sudesh K (2009) Applications of cyanobacteria in biotechnology. J Appl Microbiol 106:1–12. https://doi.org/10.1111/j.1365-2672.2008.03918
Agarwal S, Rao AV (1998) Tomato lycopene and low density lipoprotein oxidation: a human dietary intervention study. Lipids 33:981–984. https://doi.org/10.1007/s11745-998-0295-6
Albrecht M, Steiger S, Sandmann G (2001) Expression of a ketolase gene mediates the synthesis of canthaxanthin in Synechococcus leading to tolerance against photoinhibition, pigment degradation and UV-B sensitivity of photosynthesis. Photochem Photobiol 73:551–555. https://doi.org/10.1562/0031-8655(2001)0730551EOAKGM2.0.CO2
Álvarez-Gómez F, Korbee N, Casas-Arrojo V, Abdala-Díaz RT, Figueroa FL (2019) UV photoprotection, cytotoxicity and immunology capacity of red algae extracts. Molecules 24:341. https://doi.org/10.3390/molecules24020341
Andreguetti D, Stein EM, Pereira CM, Pinto E, Colepicolo P (2013) Antioxidant properties and UV absorbance pattern of mycosporine-like amino acids analogs synthesized in an environmentally friendly manner. J Biochem Mol Toxicol 27:305–312. https://doi.org/10.1002/jbt.21489
Athukorala Y, Trang S, Kwok C, Yuan YV (2016) Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 21:119. https://doi.org/10.3390/molecules21010119
Balskus EP, Walsh CT (2010) The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 329:1653–1656. https://doi.org/10.1126/science.1193637
Balskus EP, Case RJ, Walsh CT (2011) The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities. FEMS Microbiol Ecol 77:322–332. https://doi.org/10.1111/j.1574-6941.2011.01113
Barr FA, Silljé HH, Nigg EA (2004) Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol 5:429–440. https://doi.org/10.1038/nrm1401
Becker K, Hartmann A, Ganzera M, Fuchs D, Gostner JM (2016) Immunomodulatory effects of the mycosporine-like amino acids shinorine and porphyra-334. Mar Drugs 14(6):119. https://doi.org/10.3390/md14060119
Bendich A (1989) Carotenoids and the immune response. J Nutr 119(1):112–115. https://doi.org/10.1093/jn/119.1.112
Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM (2016) Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res 50:34–66. https://doi.org/10.1016/j.preteyeres.2015.10.003
Bhadury P, Wright PC (2004) Exploitation of marine algae: biogenic compounds for potential antifouling applications. Planta 219(4):561–578. https://doi.org/10.1007/s00425-004-1307-5
Bhatia S, Sharma K, Namdeo AG, Chaugule BB, Kavale M, Nanda S (2010) Broad-spectrum sunprotective action of porphyra-334 derived from Porphyra vietnamensis. Pharmacogn Res 2(1):45–49. https://doi.org/10.4103/0974-8490.60578
Bolhassani A (2017) Carotenoids and cancer: biological functions. Acta Sci Pharm Sci 1:11–20
Bultel-Poncé V, Felix-Theodore F, Sarthon C, Ponge JF, Bodo B (2004) New pigments from the terrestrial cyanobacterium Scytonema sp collected on the Mitaraka Inselberg, French Guyana. J Nat Prod 67(4):678–681. https://doi.org/10.1021/np034031u
Carignan MO, Cardozo KHM, Oliveira-Silva D, Colepicolo P, Carreto JI (2009) Palythine-threonine, a major novel mycosporine-like amino acid (MAA) isolated from the hermatypic coral Pocillopora capitata. J Photochem Photobiol B Biol 94(3):191–200. https://doi.org/10.1016/j.jphotobiol.2008.12.001
Chang J, Zhang Y, Li Y, Lu K, Shen Y, Guo Y, Qi Q, Wang M, Zhang S (2018) NrF2/ARE and NF-κB pathway regulation may be the mechanism for lutein inhibition of human breast cancer cell. Future Oncol 14(8):719–726. https://doi.org/10.2217/fon-2017-0584
Chung RWS, Leanderson P, Lundberg AK, Jonasson L (2017) Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 262:87–93. https://doi.org/10.1016/j.atherosclerosis.2017.05.008
Ciapara IH, Valenzuela LF, Goycoolea FM, Monal WA (2004) Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr Polym 56:41–45. https://doi.org/10.1016/j.carbpol.2003.11.012
Conde FR, Churio MS, Previtali CM (2004) The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem Photobiol Sci 3(10):960–967. https://doi.org/10.1039/b405782a
Crowe-White KM, Phillips TA, Ellis AC (2019) Lycopene and cognitive function. J Nutr Sci 8:e20. https://doi.org/10.1017/jns.2019.16
D’Agostino PM, Javalkote VS, Mazmouz R, Pickford R, Puranik PR, Neilan BA (2016) Comparative profiling and discovery of novel glycosylated mycosporine-like amino acids in two strains of the cyanobacterium Scytonema cf. crispum. Appl Environ Microbiol 82(19):5951–5959. https://doi.org/10.1128/AEM.01633-16
D’Agostino PM, Woodhouse JN, Liew HT, Sehnal L, Pickford R, Wong HL, Burns BP, Neilan BA (2019) Bioinformatic, phylogenetic and chemical analysis of the UV-absorbing compounds scytonemin and mycosporine-like amino acids from the microbial mat communities of Shark Bay, Australia. Environ Microbiol 21(2):702–715. https://doi.org/10.1111/1462-2920.14517
Dahms HU, Ying X, Pfeiffer C (2006) Antifouling potential of cyanobacteria: a mini-review. Biofouling 22(5–6):317–327. https://doi.org/10.1080/08927010600967261
Darvin ME, Sterry W, Lademann J, Vergou T (2011) The role of carotenoids in human skin. Molecules 16(12):10491–10506. https://doi.org/10.3390/molecules161210491
De la Coba F, Aguilera J, De Gálvez MV, Alvarez M, Gallego E, Figueroa FL, Herrera E (2009a) Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV absorbing compounds. J Dermatol Sci 55(3):161–169. https://doi.org/10.1016/j.jdermsci.2009.06.004
De la Coba F, Aguilera J, Figueroa FL, De Gálvez MV, Herrera E (2009b) Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J Appl Phycol 21:161–169
Demmig-Adams B, Adams WW III, Czygan FC, Schreiber U, Lange OL (1990) Differences in the capacity for radiation less energy dissipation in green and blue-green algal lichens associated with differences in carotenoid composition. Planta 180(4):582–589. https://doi.org/10.1007/BF02411457
Dillon JG, Castenholz RW (1999) Scytonemin, a cyanobacterial sheath pigment, protects against UVC radiation: implications for early photosynthetic life. J Phycol 35:673–681. https://doi.org/10.1046/j.1529-8817.1999.3540673
Dillon JG, Tatsumi CM, Tandingan PG, Castenholz RW (2002) Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis sp.). Arch Microbiol 177(4):322–331. https://doi.org/10.1007/s00203-001-0395
Dunlap WC, Yamamoto Y (1995) Small-molecule antioxidants in marine organisms: antioxidant activity of mycosporine-glycine. Comp Biochem Physiol B Biochem Mol Biol 112:105–114. https://doi.org/10.1016/0305-0491(95)00086
Ehling-Schulz M, Bilger W, Scherer S (1997) UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J Bacteriol 179(6):1940–1945. https://doi.org/10.1128/jb.179.6.1940-1945.1997
Frank HA, Cogdell RJ (1993) The photochemistry and functions of carotenoids in photosynthesis. In: Young A, Britton G (eds) Carotenoids in photosynthesis. Springer, London, pp 252–326. https://doi.org/10.1007/978-94-011-2124-8_8
Gacesa R, Lawrence KP, Georgakopoulos ND, Yabe K, Dunlap WC, Barlow DJ, Wells G, Young AR, Long PF (2018) The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1–Nrf2 binding. Biochimie 154:35–44. https://doi.org/10.1016/j.biochi.2018.07.020
Gao X (2017) Scytonemin plays a potential role in stabilizing the exopolysaccharidic matrix in terrestrial cyanobacteria. Microb Ecol 73(2):255–258. https://doi.org/10.1007/s00248-016-0851-4
Gao Q, Garcia-Pichel F (2011a) An ATP-grasp ligase involved in the last biosynthetic step of the iminomycosporine shinorine in Nostoc punctiforme ATCC 29133. J Bacteriol 193(21):5923–5928. https://doi.org/10.1128/JB.05730-11
Gao Q, Garcia-Pichel F (2011b) Microbial ultraviolet sunscreens. Nat Rev Microbiol 9(11):791–802. https://doi.org/10.1038/nrmicro2649
Garcia-Pichel F, Castenholz RW (1991) Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J Phycol 27:395–409. https://doi.org/10.1111/j.0022-3646.1991.00395
Garcia-Pichel F, Pringault O (2001) Cyanobacteria track water in desert soils. Nature 413(6854):380–381. https://doi.org/10.1038/35096640
Garcia-Pichel F, Sherry ND, Castenholz RW (1992) Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem Photobiol 56(1):17–23. https://doi.org/10.1111/j.1751-1097.1992.tb09596
Gierhart DL, Zeavision LLC (2019) Zeaxanthin formulations with additional ocular-active nutrients, for protecting eye health and treating eye disorders. US Patent 10,307,384 B2
Goralczyk R, Buser S, Bausch J, Bee W, Zühlke U, Barker FM (1997) Occurrence of birefringent retinal inclusions in cynomolgus monkeys after high doses of canthaxanthin. Investig Ophthalmol Vis Sci 38(3):741–752
Grant CS, Louda JW (2013) Scytonemin-imine, a mahogany-colored UV/Vis sunscreen of cyanobacteria exposed to intense solar radiation. Org Geochem 65:29–36. https://doi.org/10.1016/j.orggeochem.2013.09.014
Grether-Beck S, Marini A, Jaenicke T, Stahl W, Krutmann J (2017) Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: results from a double-blinded, placebo-controlled, crossover study. Br J Dermatol 176(5):1231–1240. https://doi.org/10.1111/bjd.15080
Grewe CB, Pulz O (2012) The biotechnology of cyanobacteria. In: Whitton B (ed) Ecology of cyanobacteria II. Springer, Dordrecht, pp 707–739. https://doi.org/10.1007/978-94-007-3855-3_26
Gröniger A, Sinha RP, Klisch M, Häder D-P (2000) Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—a database. J Photochem Photobiol B Biol 58(2–3):115–122. https://doi.org/10.1016/s1011-1344(00)00112-3
Häder D-P, Sinha RP (2005) Solar ultraviolet radiation-induced DNA damage in aquatic organisms: potential environmental impact. Mut Res 571(1–2):221–233. https://doi.org/10.1016/j.mrfmmm.2004.11.017
Hartmann A, Gostner J, Fuchs JE, Chaita E, Aligiannis N, Skaltsounis L, Ganzera M (2015) Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Med 81(10):813–820. https://doi.org/10.1055/s-0035-1546105
Huang XX, Scolyer RA, Abubakar A, Halliday GM (2012) Human 8-oxoguanine-DNA glycosylase-1 is downregulated in human basal cell carcinoma. Mol Genet Metab 106(1):127–130. https://doi.org/10.1016/j.ymgme.2012.02.017
Ibrahim K, Hassan TJ, Jafarey SN (1991) Plasma vitamin A and carotene in maternal and cord blood. Asia Oceania J Obstet Gynaecol 17(2):159–164. https://doi.org/10.1111/j.1447-0756.1991.tb00040
Ishihara K, Watanabe R, Uchida H, Suzuki T, Yamashita M, Takenaka H, Nazifi E, Matsugo S, Yamaba M, Sakamoto T (2017) Novel glycosylated mycosporine-like amino acid, 13-O-(β-galactosyl)-porphyra-334, from the edible cyanobacterium Nostoc sphaericum-protective activity on human keratinocytes from UV light. J Photochem Photobiol B Biol 172:102–108. https://doi.org/10.1016/j.jphotobiol.2017.05.019
Ito S, Hirata Y (1977) Isolation and structure of a mycosporine from the zoanthidian Palythoa tuberculosa. Tetrahedron Lett 28:2429–2430
Ito Y, Yoshida H, Matsuzuka F, Matsuura N, Nakamura Y, Nakamine H, Kakudo K, Kuma K, Miyauchi A (2004) Polo-like kinase 1 (PLK1) expression is associated with cell proliferative activity and Cdc2 expression in malignant lymphoma of the thyroid. Anticancer Res 24(1):259–264
Iwai M, Maoka T, Ikeuchi M, Takaichi S (2008) 2, 2′-β-Hydroxylase (CrtG) is involved in carotenogenesis of both nostoxanthin and 2-hydroxymyxol 2′-fucoside in Thermosynechococcus elongatus strain BP-1. Plant Cell Physiol 49(11):1678–1687. https://doi.org/10.1093/pcp/pcn142
Jiang H, Gao K, Helbling EW (2008) UV-absorbing compounds in Porphyra haitanensis (Rhodophyta) with special reference to effects of desiccation. J Appl Phycol 20:387–395. https://doi.org/10.1007/s10811-007-9268-2
Kageyama H, Waditee-Sirisattha R (2019) Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: molecular and cellular mechanisms in the protection of skin-aging. Mar Drugs 17(4):222. https://doi.org/10.3390/md17040222
Kang MR, Jo SA, Lee H, Yoon YD, Kwon JH, Yang JW, Choi BJ, Park KH, Lee MY, Lee CW, Lee KR (2020) Inhibition of skin inflammation by scytonemin, an ultraviolet sunscreen pigment. Mar Drugs 18(6):300. https://doi.org/10.3390/md18060300
Karentz D, McEuen FS, Land MC, Dunlap WC (1991) Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: potential protection from ultraviolet exposure. Mar Biol 108:157–166
Kaur G, Sandal A, Dhillon NS (2017) Lycopene and human health—a review. Agric Rev 38:282–289. https://doi.org/10.18805/ag.R-1741
Kawata A, Murakami Y, Suzuki S, Fujisawa S (2018) Anti-inflammatory activity of β-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 32(2):255–264. https://doi.org/10.21873/invivo.11232
Klaui H (1982) Industrial and commercial uses of carotenoids. In: Britton G, Goodwin TW (eds) IUPAC carotenoid chemistry and biochemistry. Pergamon Press, Oxford, pp 309–317
Kobayashi J, Nakamura N, Hirata Y (1981) Isolation and structure of a UV-absorbing substance 337 from the ascidian Halocynthia roretzi. Tetrahedron Lett 22:3001–3002. https://doi.org/10.1016/S0040-4039(01)81811-6
Kollera M, Salernob A, Brauneggc G (2015) Value-added products from algal biomass. In: Perosa A, Guido B, Ravagnan G, Zinoviev S (eds) Algae as a potential source of food and energy in developing countries, Edizioni Ca’ Foscari, Venezia, Italia, p 19
Kornhauser A, Wamer W, Lambert L (1990). In: Krinsky NI, Mathews-Roth MM, Taylor RF (eds) Carotenoids: chemistry and biology. Plenum Press, New York, pp 301–312
Kosourov S, Murukesan G, Jokela J, Allahverdiyeva Y (2016) Carotenoid biosynthesis in Calothrix sp. 336/3: composition of carotenoids on full medium, during diazotrophic growth and after long-term H2 photoproduction. Plant Cell Physiol 57(11):2269–2282. https://doi.org/10.1093/pcp/pcw143
Kusama Y, Inoue S, Jimbo H, Takaichi S, Sonoike K, Hihara Y, Nishiyama Y (2015) Zeaxanthin and echinenone protect the repair of photosystem II from inhibition by singlet oxygen in Synechocystis sp. PCC 6803. Plant Cell Physiol 56(5):906–916. https://doi.org/10.1093/pcp/pcv018
Lawrence KP, Long PF, Young AR (2018) Mycosporine-like amino acids for skin photoprotection. Curr Med Chem 25(40):5512–5527. https://doi.org/10.2174/0929867324666170529124237
Lee EH, Faulhaber D, Hanson KM, Ding W, Peters S, Kodali S, Granstein RD (2004) Dietary lutein reduces ultraviolet radiation-induced inflammation and immunosuppression. J Investig Dermatol 122(2):510–517. https://doi.org/10.1046/j.0022-202X.2004.22227
Lisby S, Gniadecki R, Wulf HC (2005) UV-induced DNA damage in human keratinocytes: quantitation and correlation with long-term survival. Exp Dermatol 14(5):349–355. https://doi.org/10.1111/j.0906-6705.2005.00282
Llewellyn CA, Airs RL, Farnham G, Greig C (2020) Synthesis, regulation and degradation of carotenoids under low level UV-B radiation in the filamentous cyanobacterium Chlorogloeopsis fritschii PCC 6912. Front Microbiol 11:163. https://doi.org/10.3389/fmicb.2020.00163
Lunel M-C, Arpin N, Favre-Bonvin J (1980) Structure of nor-mycosporin glutamine, a new compound isolated from Pyronema omphalodes (bull ex fr.) fuckel. Tetrahedron Lett 21:4715–4716. https://doi.org/10.1016/0040-4039(80)88101-9
Mathews-Roth MM (1990) Plasma concentrations of carotenoids after large doses of beta-carotene. Am J Clin Nutr 52(3):500–501. https://doi.org/10.1093/ajcn/52.3.500
Matsui K, Nazifi E, Kunita S, Wada N, Matsugo S, Sakamoto T (2011) Novel glycocylated mycosporine-like amino acids with radical scavenging activity from the cyanobacterium Nostoc commune. J Photochem Photobiol B Biol 105(1):81–89. https://doi.org/10.1016/j.jphotobiol.2011.07.003
Matsui K, Nazifi E, Hirai Y, Wada N, Matsugo S, Sakamoto T (2012) The cyanobacterial UV-absorbing pigment scytonemin displays radical scavenging activity. J Gen Appl Microbiol 58(2):137–144. https://doi.org/10.2323/jgam.58.137
McAdam E, Brem R, Karran P (2016) Oxidative stress-induced protein damage inhibits DNA repair and determines mutation risk and therapeutic efficacy. Mol Cancer Res 14(7):612–622. https://doi.org/10.1158/1541-7786.MCR-16-0053
McInnes C, Mezna M, Fischer PM (2005) Progress in the discovery of polo-like kinase inhibitors. Curr Top Med Chem 5(2):181–197. https://doi.org/10.2174/1568026053507660
Melis A (2012) Photosynthesis-to-fuels: from sunlight to hydrogen, isoprene, and botryococcene production. Energy Environ Sci 5:5531–5539
Mercurio DG, Wagemaker TAL, Alves VM, Benevenuto CG, Gaspar LR, Campos PM (2015) In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extract. J Photochem Photobiol B Biol 153:121–126. https://doi.org/10.1016/j.jphotobiol.2015.09.016
Merhan O (2017) Biochemistry and antioxidant properties of carotenoids. Carotenoids 5:51. https://doi.org/10.5772/67592
Mishra A, Tandon R, Kesarwani S, Singh R, Tiwari GL (2015) Emerging applications of cyanobacterial ultraviolet protecting compound scytonemin. J Appl Phycol 27:1045–1051. https://doi.org/10.1007/s10811-014-0399
Mishra S, Pandey A, Ahmed H, Kumar D, Singh V, Sinha RP (2019a) Mycosporine-like amino acids (MAAs) profile of cyanobacteria from different historical kunds of Varanasi, India. Int J Appl Sci Biol 7:317–326. https://doi.org/10.3126/ijasbt.v7i3.25712
Mishra S, Pandey A, Sinha RP (2019b) Characterization of photoprotective and pharmaceutically important compounds from cyanobacteria and algal assemblages from historical kunds of Varanasi, India. Chem Pharm Res 11(9):24–36
Misonou T, Saitoh J, Oshiba S, Tokitomo Y, Maegawa M, Inoue Y, Sakurai T (2003) UV-absorbing substance in the red alga Porphyra yezoensis (bangiales, rhodophyta) block thymine photodimer production. Mar Biotechnol 5(2):194–200. https://doi.org/10.1007/s10126-002-0065-2
Nakamura H, Kobayashi J, Hirata Y (1981) Isolation and structure of a 330 nm UV-absorbing substance, asterina-330 from the starfish Asterina pectinifera. Chem Lett 28:1413–1414. https://doi.org/10.1246/cl.1981.1413
Nakamura H, Kobayashi J, Hirata Y (1982) Separation of mycosporine-like amino acids in marine organisms using reversed-phase high-performance liquid chromatography. J Chromatogr A 250:113–118. https://doi.org/10.1016/S0021-9673(00)95219-1
Nazifi E, Wada N, Yamaba M, Asano T, Nishiuchi T, Matsugo S, Sakamoto T (2013) Glycosylated porphyra-334 and palythine-threnonine from the terrestrial cyanobacterium Nostoc commune. Mar Drugs 11(9):3124–3154. https://doi.org/10.3390/md11093124
Nazifi E, Wada N, Asano T, Nishiuchi T, Iwamuro Y, Chinaka S, Matsugo S, Sakamoto T (2015) Characterization of the chemical diversity of glycosylated mycosporine-like amino acids in the terrestrial cyanobacterium Nostoc commune. J Photochem Photobiol B Biol 142:154–168. https://doi.org/10.1016/j.jphotobiol.2014.12.008
Nishino H (1998) Cancer prevention by carotenoids. Mut Res 402(1–2):59–163. https://doi.org/10.1016/s0027-5107(97)00293-5
O’Connor C, Skill SC, Llewellyn CA (2011) Cosmetic attributes of algae—a review. Topical composition PCT/GB2011/051138
Oliver JWK, Atsumi S (2014) Metabolic design for cyanobacterial chemical synthesis. Photosynth Res 120(3):249–261. https://doi.org/10.1007/s11120-014-9997-4
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269(1):1–10. https://doi.org/10.1111/j.1574-6968.2007.00650
Osawa A, Harada H, Choi S-K, Misawa N, Shindo K (2011) Production of caloxanthin 3′-β-d-glucoside, zeaxanthin 3,3′-β-d-diglucoside, and nostoxanthin in a recombinant Escherichia coli expressing system harboring seven carotenoid biosynthesis genes, including crtX and crtG. Phytochemical 72(8):711–716. https://doi.org/10.1016/j.phytochem.2011.02.017
Oyamada C, Kaneniwa M, Ebitani K, Murata M, Ishihara K (2008) Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Mar Biotechnol 10(2):41–150. https://doi.org/10.1007/s10126-007-9043
Paliwal C, Ghosh T, Bhayani K, Maurya R, Mishra S (2015) Antioxidant, anti-nephrolithe activities and in vitro digestibility studies of three different cyanobacterial pigment extracts. Mar Drugs 13(8):5384–5401. https://doi.org/10.3390/md13085384
Pereira M, Pereira N, Rosado C, de Oliveira CA, Peres DA, Araújo ME, Velasco MVR, Baby AR, Mota J, Almeida TS (2015) Photostabilization of sunscreens by incorporation of tea as the external phase. Biomed Biopharm Res 12:107–116
Peres DA, De Oliveira CA, Da Costa MS, Tokunaga VK, Mota JP, Rosado C, Consiglieri VO, Kaneko TM, Velasco MVR, Baby AR (2015) Rutin increases critical wavelength of systems containing a single UV filter and with good skin compatibility. Skin Res Technol 22(3):325–333. https://doi.org/10.1111/srt.12265
Petersen B, Datta P, Philipsen PA, Wulf HC (2013) Sunscreen use and failures-on site observations on a sun holiday. Photochem Photobiol Sci 12(1):90–196. https://doi.org/10.1039/c2pp25127b
Pfeifer GP, Besaratinia A (2012) UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci 11(1):90–97. https://doi.org/10.1039/c1pp05144j
Piiparinen J, Enberg S, Rintala J-M, Sommaruga R, Majaneva M, Autio R, Vahatalo AV (2015) The contribution of mycosporine-like amino acids, chromophoric dissolved organic matter and particles to the UV of sea-ice organisms in the Baltic Sea. Photochem Photobiol Sci 14(5):1025–1038. https://doi.org/10.1039/c4pp00342j
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ (2009) Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc 14(1):20–24. https://doi.org/10.1038/jidsymp.2009.8
Rao CSVR (1994) Antimicrobial activity of cyanobacteria. Indian J Mar Sci 23:55–56
Rastogi RP, Incharoensakdi A (2013) UVR-induced accumulation of photoprotective compounds in the green alga Tetraspora sp. CU2551. Plant Physiol Biochem 70:7–13. https://doi.org/10.1016/j.plaphy.2013.04.021
Rastogi RP, Incharoensakdi A (2014) Characterization of UV screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol Ecol 87(1):244–256. https://doi.org/10.1111/1574-6941.12220
Rastogi RP, Sinha RP (2009) Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv 27(4):521–539. https://doi.org/10.1016/j.biotechadv.2009.04.009
Rastogi RP, Richa SRP, Singh SP, Häder D-P (2010) Photoprotective compounds from marine organisms. J Ind Microbiol Biotechnol 37(6):537–558. https://doi.org/10.1007/s10295-010-0718-5
Rastogi RP, Sonani RR, Madamwar D (2014) The high-energy radiation protectant extracellular sheath pigment scytonemin and its reduced counterpart in the cyanobacterium Scytonema sp. R77DM. Bioresour Technol 171:396–400. https://doi.org/10.1016/j.biortech.2014.08.106
Rastogi RP, Madamwar D, Incharoensakdi A (2015a) Sunscreening bioactive compounds mycosporine-like amino acids in naturally occurring cyanobacterial biofilms: role in photoprotection. J Appl Microbiol 11:753–762. https://doi.org/10.1111/jam.12879
Rastogi RP, Sonani RR, Madamwar D (2015b) Cyanobacterial Sunscreen scytonemin: role in photoprotection and biomedical research. Appl Biochem Biotechnol 176(6):1551–1563. https://doi.org/10.1007/s12010-015-1676-1
Rastogi RP, Sonani RR, Madamwar D, Incharoensakdi A (2016) Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res 16:110–118. https://doi.org/10.1016/j.algal.2016.03.009
Rastogi RP, Sinha RP, Incharoensakdi A (2013) Partial characterization, UV-induction and photoprotective function of sunscreen pigment, scytonemin from Rivularia sp. HKAR-4. Chemosphere 93(9):1874–1878. https://doi.org/10.1016/j.chemosphere.2013.06.057
Řezanka T, Temina M, Tolstikov AG, Dembitsky VM (2004) Natural microbial UVR filters mycosporine like amino acids. Folia Microbiol (Praha) 49(4):339–352. https://doi.org/10.1007/BF03354663
Richa, Sinha RP (2015) Biochemical characterization of sunscreening mycosporine-like amino acids from two Nostoc species inhabiting diverse habitats. Protoplasma 252:199–208. https://doi.org/10.1007/s00709-014-0674-4
Richa, Rastogi RP, Kumari S, Singh KL, Kannaujiya VK, Singh G, Keshari M, Sinha RP (2011) Biotechnological potential of mycosporine-like amino acids and phycobiliproteins of cyanobacterial origin. Biotechnol Bioinform Bioeng 1:159–171
Rojas J, Londoño C, Ciro Y (2016) The health benefits of natural skin UVA photoprotective compounds found in botanical sources. Int J Pharm Sci 8:13–23
Roullier C, Chollet-Krugler M, Pferschy-Wenzig EM, Maillard A, Rechberger GN, Legouin-Gargadennec B, Bauer R, Boustie J (2011) Characterization and identification of mycosporines-like compounds in cyanolichens. Isolation of mycosporine hydroxyglutamicol from Nephroma laevigatum Ach. Phytochemical 72(11–12):1348–1357. https://doi.org/10.1016/j.phytochem.2011.04.002
Russell RM (2002) Beta-carotene and lung cancer. Pure Appl Chem 74:1461–1467. https://doi.org/10.1351/pac200274081461
Ryu J, Park SJ, Kim IH, Choi YH, Nam TJ (2014) Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int J Mol Med 34(3):796–803. https://doi.org/10.3892/ijmm.2014.1815
Ryu J, Kwon MJ, Nam TJ (2015) Nrf2 and NF-κB signaling pathways contribute to porphyra-334-mediated inhibition of UVA-induced inflammation in skin fibroblasts. Mar Drugs 13(8):4721–4732. https://doi.org/10.3390/md13084721
Sekikawa I, Kubota C, Hiraoki T, Tsujino I (1986) Isolation and structure of a 357 nm UV-absorbing substance, usujirene, from the red alga Palmaria palmata (L.) O. Kuntze. Jpn J Phycol 34:185–188
Singh G, Kumar J (2018) Artificial and natural photoprotective compounds. In: Rastogi RP (ed) Sunscreens: source, formulations, efficacy and recommendations. Nova Science Publishers, Hauppauge, pp 153–200
Singh SP, Kumari S, Rastogi RP, Singh KL, Sinha RP (2008a) Mycosporine-like amino acids (MAAs): chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J Exp Biol 46(1):7–17
Singh SP, Sinha RP, Klisch M, Häder D-P (2008b) Mycosporine-like amino acids (MAAs) profile of a rice-field cyanobacterium Anabaena doliolum as influenced by PAR and UVR. Planta 229(1):225–233. https://doi.org/10.1007/s00425-008-0822-1
Singh SP, Kumari S, Rastogi RP, Singh KL, Richa SRP (2010) Photoprotective and biotechnological potentials of cyanobacterial sheath pigment, scytonemin. Afr J Biotechnol 9:580–588. https://doi.org/10.5897/AJB09.019
Sinha RP, Klisch M, Gröniger A, Häder D-P (2000) Mycosporine-like amino acids in the marine red alga Gracilaria cornea—effects of UV and heat. Environ Exp Bot 43:33–43. https://doi.org/10.1016/S0098-8472(99)00043-X
Sinha RP, Singh SP, Häder D-P (2007) Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J Photochem Photobiol B Biol 89(1):29–35. https://doi.org/10.1016/j.jphotobiol.2007.07.006
Soule T, Palmer K, Gao Q, Potrafka RM, Stout V, Garcia-Pichel F (2009) A comparative genomics approach to understanding the biosynthesis of the sunscreen scytonemin in cyanobacteria. BMC Genom 10:336. https://doi.org/10.1186/1471-2164-10-336
Squier AH, Airs RL, Hodgson DA, Keely BJ (2004a) Atmospheric pressure chemical ionisation liquid chromatography/mass spectrometry of the ultraviolet screening pigment scytonemin: characteristic fragmentations. Rap Commun Mass Spectrom 18(23):2934–2938. https://doi.org/10.1002/rcm.1714
Squier AH, Hodgson DA, Keely BJ (2004b) A critical assessment of the analysis and distributions of scytonemin and related UV screening pigments in sediments. Org Geochem 35:1221–1228. https://doi.org/10.1016/j.orggeochem.2004.07.005
Stahl W, Sies H (2005) Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta 1740(2):101–107. https://doi.org/10.1016/j.bbadis.2004.12.006
Stochaj WR, Dunlap WC, Shick JM (1994) Two new UV absorbing mycosporine-like amino acids from the sea anemone Anthopleura elegantissima and the effects of zooxanthellae and spectral irradiance on chemical composition and content. Mar Biol 118:149–156
Strebhardt K, Ullrich A (2006) Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer 6(4):321–330. https://doi.org/10.1038/nrc1841
Stringham JM, Hammond BR Jr (2005) Dietary lutein and zeaxanthin: possible effects on visual function. Nutr Rev 63(2):59–64. https://doi.org/10.1111/j.1753-4887.2005.tb00122
Suh HJ, Lee HW, Jung J (2003) Mycosporine glycine protects biological systems against photodynamic damage by quenching singlet oxygen with a high efficiency. Photochem Photobiol 78(2):109–113. https://doi.org/10.1562/0031-8655(2003)078%3c0109:mgpbsa%3e2.0.co;2
Suh SS, Hwang J, Park M, Seo HH, Kim HS, Lee JH, Moh SH, Lee TK (2014) Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar Drugs 12(10):5174–5187. https://doi.org/10.3390/md12105174
Takano S, Uemura D, Hirata Y (1978) Isolation and structure of two new amino acids, palythinol and palythene, from the zoanthid Palythoa tuberculosa. Tetrahedron Lett 49:4909–4912. https://doi.org/10.1016/S0040-4039(01)85768-3
Takano S, Nakanishi A, Uemura D, Hirata Y (1979) Isolation and structure of a 334 nm UV-absorbing substance, porphyra-334 from the red alga Porphyra tenera Kjellman. Chem Lett 4:419–420. https://doi.org/10.1246/cl.1979.419
Tanaka T, Shnimizu M, Moriwaki H (2012) Cancer chemoprevention by carotenoids. Molecules 17(3):3202–3242. https://doi.org/10.3390/molecules17033202
Tarasuntisuk S, Palaga T, Kageyama H, Waditee-Sirisattha R (2019) Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated raw 264.7 macrophages. Arch Biochem Biophys 662:33–39. https://doi.org/10.1016/j.abb.2018.11.026
Teai TT, Raharivelomanana P, Bianchini J-P, Faure R, Martin PMV, Cambon A (1997) Structure de deux nouvelles iminomycosporines isolées de Pocillopora eydouxi. Tetrahedron Lett 38:5799–5800. https://doi.org/10.1016/S0040-4039(97)01281-1
Tsujino I, Yabe K, Sekikawa I (1980) Isolation and structure of a new amino acid, shinorine, from the red alga Chondrus yendoi Yamada et Mikami. Bot Mar 23:65–68
Tuzcu M, Orhan C, Muz OE, Sahin N, Juturu V, Sahin K (2017) Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol 17(1):129. https://doi.org/10.1186/s12886-017-0524-1
Valencia A, Kochevar IE (2008) Nox1-based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. J Investig Dermatol 128(1):214–222. https://doi.org/10.1038/sj.jid.5700960
Varnali T, Edwards HGM (2013) Theoretical study of novel complexed structures for methoxy derivatives of scytonemin: potential biomarkers in iron-rich stressed environments. Astrobiology 13(9):861–869. https://doi.org/10.1089/ast.2013.0980
Varnali T, Edwards HGM (2014) Scytonin, a novel cyanobacterial photoprotective pigment: calculations of raman spectroscopic biosignatures. J Mol Model 20(3):2157. https://doi.org/10.1007/s00894-014-2157-0
Volkmann M, Gorbushina AA, Kedar L, Oren A (2006) Structure of euhalothece-362, a novel red-shifted mycosporine-like amino acid, from a halophilic cyanobacterium (Euhalothece sp.). FEMS Microbiol Lett 258(1):50–54. https://doi.org/10.1111/j.1574-6968.2006.00203
Wada N, Sakamoto T, Matsugo S (2013) Multipleroles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 3(2):463–483. https://doi.org/10.3390/metabo3020463
Wada N, Sakamoto T, Matsugo S (2015) Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 4(3):603–646. https://doi.org/10.3390/antiox4030603
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29(2):949–982. https://doi.org/10.1007/s10811-016-0974-5
Whitehead K, Hedges JI (2002) Analysis of mycosporine-like amino acids in plankton by liquid chromatography electrospray ionization mass spectrometry. Mar Chem 80:27–39
Whitehead K, Hedges JI (2005) Photodegradation and photosensitization of mycosporine-like amino acids. J Photochem Photobiol B Biol 80(2):115–121. https://doi.org/10.1016/j.jphotobiol.2005.03.008
Wisniewska A, Subczynski WK (1998) Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochim Biophys Acta Biomembr 1368(2):235–246. https://doi.org/10.1016/s0005-2736(97)00182
Wölfle U, Seelinger G, Bauer G, Meinke MC, Lademann J, Schempp CM (2014) Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol Physiol 27(6):316–332. https://doi.org/10.1159/000360092
Xie Z, Wang Y, Liu Y, Liu Y (2009) Ultraviolet-B exposure induces photo-oxidative damage and subsequent repair strategies in a desert cyanobacterium Microcoleus vaginatus Gom. Eur J Soil Biol 45(4):377–382. https://doi.org/10.1016/j.ejsobi.2009.04.003
Yang C-M, Lu Y-L, Chen H-Y, Hu M-L (2012) Lycopene and the LXRα agonist T0901317 synergistically inhibit the proliferation of androgen-independent prostate cancer cells via the PPARγ–LXRα–ABCA1 pathway. J Nutr Biochem 23(9):1155–1162. https://doi.org/10.1016/j.jnutbio.2011.06.009
Yang G, Cozad MA, Holland DA, Zhang Y, Luesch H, Ding Y (2018) Photosynthetic production of sunscreen shinorine using an engineered cyanobacterium. ACS Syn Biol 7(2):664–671. https://doi.org/10.1021/acssynbio.7b00397
Young H, Patterson VJ (1982) A UV-protective compound from Glomerella cingulata—a mycosporine. Phytochem 21:1075–1077. https://doi.org/10.1016/S0031-9422(00)82419
Young AR, Chadwick CA, Harrison GI, Nikaido O, Ramsden J, Potten CS (1998) The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J Investig Dermatol 111(6):982–988. https://doi.org/10.1046/j.1523-1747.1998.00436
Yuan YV, Carrington MF, Walsh NA (2005) Extracts from dulse (Palmaria palmata) are effective antioxidants and inhibitors of cell proliferation in vitro. Food Chem Toxicol 43(7):1073–1081. https://doi.org/10.1016/j.fct.2005.02.012
Zhang G, Zhang Z, Liu Z (2013) Scytonemin inhibits cell proliferation and arrests cell cycle through down regulating Plk1 activity in multiple myeloma cells. Tumour Biol 34(4):2241–2247. https://doi.org/10.1007/s13277-013-0764-5
Acknowledgements
S.M. is thankful to University Grants Commission (UGC), New Delhi, India, for the financial assistance in the form of fellowship as Junior Research Fellow (Joint CSIR-UGC JRF-2019/NTA Ref. No.: 191620046790).
Author Information
Laboratory of Photobiology and Molecular Microbiology, Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi, India
Laboratory of Photobiology and Molecular Microbiology, Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi, India
rpsinhabhu@gmail.com