Azospirillum brasilense mitigates anatomical alterations produced by salt stress in jojoba in vitro plants
Research Articles | Published: 03 August, 2021
Online ISSN : 2229-4473.
Website:www.vegetosindia.org
Pub Email: contact@vegetosindia.org
First Page: 725
Last Page: 737
Views: 1888
Keywords:
Azospirillum brasilense
, In vitro culture, Plant anatomy, Plant growth promoting rhizobacteria, Salt stress,
Simmondsia chinensis
Abstract
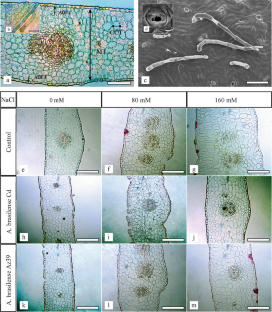
Jojoba, Simmondsia chinensis (Link) Schneider, is a slow-growing woody perennial shrub, whose production can be limited by soil salinity. Based on the fact that alterations of salinity stress can be reduced by biofertilization, the aim of this work was to study the effects of salinity and Azospirillum brasilense inoculation on the probable structural characteristics of leaves, stems, and roots of jojoba plants grown in vitro by light and scanning electron microscopy. Salt stress induces changes in anatomical characteristics of jojoba in vitro such as increment in mesophyll thickness on leaves, and increases in the cortex, pith, and xylem vessel diameters on stems. Cell density decreased in leaf and stem, and increased in roots. Azospirillum brasilense inoculations mitigated leaf succulence and prevent the root anatomical alterations caused by salinity displaying leaf mesophyll and chlorenchyma thickness, root diameter, cortex thickness, and vascular bundle diameter similar values to control plants in some treatments. Also improved the anatomical characteristic of the stem and increment the xylem vessels ratio between stem and root. The anatomical changes induced by A. brasilense could protect jojoba plants from the detrimental effects of saline stress and explain the higher tolerance to salinity of inoculated plants.
(*Only SPR Members can get full access. Click Here to Apply and get access)
References
Acosta-Motos J, Ortuño M, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco M, Hernandez J (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18. https://doi.org/10.3390/agronomy7010018
Akcin TA, Akcin A, Yalcın E (2017) Anatomical changes induced by salinity stress in Salicornia freitagii (Amaranthaceae). Braz J Bot 40:1013–1018. https://doi.org/10.1007/s40415-017-0393-0
Al-Obaidi JR, Halabi MF, AlKhalifah NS, Asanar S, Al-Soqeer AA, Attia MF (2017) A review on plant importance, biotechnological aspects, and cultivation challenges of jojoba plant. Biol Res 50:5. https://doi.org/10.1186/s40659-017-0131-x
Apóstolo NM, Llorente BE (2000) Anatomy of normal and hyperhydric leaves and shoots of in vitro grown Simmondsia chinensis (Link) Schn. Vitro Cell Dev Plant 36:243–249. https://doi.org/10.1007/s11627-000-0045-z
Arias C, Serrat X, Moysset L, Perissé P, Nogués S (2018) Morpho-physiological responses of alamo switchgrass during germination and early seedling stage under salinity or water stress conditions. Bioenergy Res 11:677–688. https://doi.org/10.1007/s12155-018-9930-3
Benzioni A (1988) Water status and its control in jojoba (Simmondsia chinensis L.). In: Proceedings of the VII international conference on jojoba and its uses. American Oil Chemist Society, Champaign, IL, USA, pp 20–35
Benzioni A, Nerd A, Rosenärtner Y, Mills D (1992) Effect of NaCl salinity on growth and development of jojoba clones: I. Young plants. J Plant Physiol 139:731–736. https://doi.org/10.1016/S0176-1617(11)81719-0
Berrichi A, Tazi R, Bellirou A, Kouddane N, Bouali A (2010) Role of salt stress on seed germination and growth of jojoba plant Simmondsia chinensis (Link) Schneider. IUFS J Biol 69:33–39
Botti C, Palzkill D, Munoz D, Prat L (1998) Morphological and anatomical characterization of six jojoba clones at saline and non-saline sites. Ind Crops Prod 9:53–62. https://doi.org/10.1016/S0926-6690(98)00014-4
Boughalleb F, Denden M, Tiba BB (2009) Anatomical changes induced by increasing NaCl salinity in three fodder shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea. Acta Physiol Plant 31:947–960. https://doi.org/10.1007/s11738-009-0310-7
Céccoli G, Ramos JC, Ortega LI, Acosta JM, Perreta MG (2011) Salinity induced anatomical and morphological changes in Chloris gayana Kunth roots. Biocell 35:9–17. https://doi.org/10.36204/biocell.2011.35.009
Chakdar H, Borse DN, Verma S, Choudhary P, Das S (2019) Microbial management of crop salinity stress: mechanisms, applications, and prospects. In: Akhtar M (ed) Salt stress, microbes, and plant interactions: mechanisms and molecular approaches, vol 2. Springer, Singapore, pp 1–25. https://doi.org/10.1007/978-981-13-8805-7
Dolatabadian A, Modarres-Sanavy SAM, Ghanati F (2011) Effect of salinity on growth, xylem structure and anatomical characteristics of soybean. Not Sci Biol 3:41–45. https://doi.org/10.15835/nsb315627
El-Afry MM, El-Nady MF, Abdelmonteleb EB, Metwaly MM (2012) Anatomical studies on drought-stressed wheat plants (Triticum aestivum L.) treated with some bacterial strains. Acta Biol Szeged 56:165–174
Gonzalez AJ, Larraburu EE, Llorente BE (2015) Azospirillum brasilense increased salt tolerance of jojoba during in vitro rooting. Ind Crops Prod 76:41–48. https://doi.org/10.1016/j.indcrop.2015.06.017
Hameed M, Ashraf M, Ahmad MS, Naz N (2010) Structural and functional adaptations in plants for salinity tolerance. In: Ashraf M, Ozturk M, Ahmad M (eds) Plant adaptation and phytoremediation. Springer, Dordrecht, pp 151–170. https://doi.org/10.1007/978-90-481-9370-7_8
Ishikawa T, Shabala S (2018) Control of xylem Na+ loading and transport to the shoot in rice and barley as a determinant of differential salinity stress tolerance. Physiol Plant 165:619–631. https://doi.org/10.1111/ppl.12758
Jafarian T, Zarea MJ, Siosemardeh A (2017) Histological responses of two wheat species to Azospirillum inoculation under dryland farming. J Plant Physiol Breed 7:67–79
Jha D, Kulshreshtha S, Chauhan S (2020) Plant microbial ecology as a potential option for stress management in plants. In: Varma A (ed) Plant microbe symbiosis Ch 17. Springer, Cham, pp 331–360. https://doi.org/10.1007/978-3-030-36248-5_17
Khairi MMA (2019) Genetics and breeding of jojoba [Simmondsia chinensis (Link) Schneider]. In: Al-Khayri J, Jain S, Johnson D (eds) Advances in plant breeding strategies: industrial and food crops. Springer, Cham. https://doi.org/10.1007/978-3-030-23265-8_8
Larraburu EE, Llorente BE (2015) Azospirillum brasilense enhances in vitro rhizogenesis of Handroanthus impetiginosus (pink lapacho) in different culture media. Ann for Sci 72:219–229. https://doi.org/10.1007/s13595-014-0418-9
Larraburu EE, Bususcovich AC, Llorente BE (2016) Azospirillum brasilense improves in vitro and ex vitro rooting-acclimatization of jojoba. Sci Hortic 209:139–147. https://doi.org/10.1016/j.scienta.2016.06.016
Llorente BE, Apóstolo NM (2013) In vitro propagation of jojoba. In: Lambardi M, Ozudogru EA, Jain SM (eds) Protocols for micropropagation of selected economically-important horticultural plants. Humana Press, New York, pp 19–31. https://doi.org/10.1007/978-1-62703-074-8_2
Mills D, Zhang G, Benzioni A (2001) Effect of different salt and of ABA on growth and mineral uptake in jojoba shoots grown in vitro. J Plant Physiol 158:1031–1039. https://doi.org/10.1078/0176-1617-00254
Naseer M, Hameed M, Zahoor A, Ahmad F, Fatima S, Ahmad MS et al (2017) Photosynthetic response in buttonwood (Conocarpus erectus L.) to salt stress. Pak J Bot 49:847–856
Orlikowska T, Nowak K, Reed B (2017) Bacteria in the plant tissue culture environment. Plant Cell Tissue Org 128(3):487–508. https://doi.org/10.1007/s11240-016-1144-9
Paradiso R, Arena C, De Micco V, Giordano M, Aronne G, De Pascale S (2017) Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front Plant Sci 8:674. https://doi.org/10.3389/fpls.2017.00674
Rodríguez-Cáceres EA (1982) Improved medium for isolation of Azospirillum spp. Appl Environ Microbiol 44:990–991
Romero AM, Vega D, Correa OS (2014) Azospirillum brasilense mitigates water stress imposed by a vascular disease by increasing xylem vessel area and stem hydraulic conductivity in tomato. Appl Soil Ecol 82:38–43. https://doi.org/10.1016/j.apsoil.2014.05.010
Roussos PA, Tsantili E, Pontikis CA (2006) Responses of jojoba explants to different salinity levels during the proliferation stage in vitro. Ind Crops Prod 23:65–72. https://doi.org/10.1016/j.indcrop.2005.04.006
Roussos PA, Gasparatos D, Tsantili E, Pontikis CA (2007) Mineral nutrition of jojoba explants in vitro under sodium chloride salinity. Sci Hortic 114:59–66. https://doi.org/10.1016/j.scienta.2007.05.001
Shabala S, Hariadi Y, Jacobsen SE (2013) Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J Plant Physiol 170(10):906–914. https://doi.org/10.1016/j.jplph.2013.01.014
Venturas MD, Sperry JS, Hacke UG (2017) Plant xylem hydraulics: what we understand, current research, and future challenges. J Integr Plant Biol 59:356–389. https://doi.org/10.1111/jipb.12534
Yermanos DM, Francois LE, Tammadoni T (1967) Effects of soil salinity on the development of jojoba. Econ Bot 21:69–80
Zarlavsky GE (2014) Histología Vegetal: Técnicas simples y complejas. Sociedad Argentina de Botanica, Buenos Aires
Zhao S, Wei H, Lin CY, Zeng Y, Tucker MP, Himmel ME, Ding SY (2016) Burkholderia phytofirmans inoculation-induced changes on the shoot cell anatomy and iron accumulation reveal novel components of Arabidopsis–Endophyte interaction that can benefit downstream biomass deconstruction. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00024
Acknowledgements
This research was supported by a grant from Departamento de Ciencias Básicas, Universidad Nacional de Luján, Luján (Bs As) Argentina (Grant number Disposición CD-CB Nº 540/18). We thank Dr. Yaacov Okon (Faculty of Agriculture, the Hebrew University of Jerusalem, Israel), Ing. Enrique Rodríguez Cáceres (National Agricultural Technology Institute (INTA), Castelar, Argentina) for kindly providing A. brasilense Cd and A. brasilense Az39 and Ing. NorbertoVinelli (La Semillera Riojana, La Rioja, Argentina) for kindly providing plant material.
Author Information
Laboratorio de Cultivo de Tejidos Vegetales, Departamento de Ciencias Básicas, Universidad Nacional de Luján, Luján, Argentina
Laboratorio de Cultivo de Tejidos Vegetales, Departamento de Ciencias Básicas, Universidad Nacional de Luján, Luján, Argentina
ezequiel.e.larraburu@gmail.com