High frequency in vitro callogenesis and plant regeneration of Glycyrrhiza glabra L.
Research Articles | Published: 27 May, 2021
Online ISSN : 2229-4473.
Website:www.vegetosindia.org
Pub Email: contact@vegetosindia.org
First Page: 495
Last Page: 504
Views: 1845
Keywords:
Browning, Callus,
Glycyrrhiza glabra L., Licorice, Phytohormone, Regeneration
Abstract
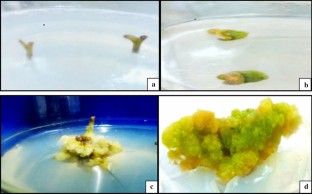
Glycyrrhiza glabra, member of Leguminosae family, is economically valuable due to its medicinal properties. Today it is considered as an endangered plant, therefore establishing a rapid and efficient system for micropropagation of this plant is desirable for their protection. The present study standardized the micropropagation protocol for G. glabra involving callus induction and shoots regeneration with explants viz. leaf and stem. Our finding suggests that the use of only one sterilant is not efficient for the sterilization of licorice explant. HgCl2 (0.1%) for 5 min and ethanol (70%) for 5 min was found best when used along with prior washing and surface sterilization with tween-20 and bavistin. Callus induction was achieved on MS medium containing various concentration of BAP, 2,4-D and NAA. Maximum callus induction was observed on MS medium containing 2 mg/l BAP, 0.5 mg/l 2,4-D and 50 mg/l ascorbic acid with both leaf and stem explants. Production of callus using leaf as an explant was better than that of stem. The development of shoots from callus was initiated on MS medium containing BAP and IAA. The most efficient shoot formation was observed with 4 mg/l BAP, 0.2 mg/l IAA and 60 mg/l ascorbic acid. Best rooting was recorded with 3 mg/l IBA. Histological aspect of indirect organogenesis of G. glabra were evaluated. Successful multiplication of shoots from explants leads to the production of improved quality and great quantity of planting material.
(*Only SPR Members can get full access. Click Here to Apply and get access)
References
- Abdelwahd R, Hakam N, Labhilili M, Udupa S (2008) Use of an adsorbent and antioxidants to reduce the effects of leached phenolics in in vitro plantlet regeneration of faba bean. Afr J Biotechnol 7(8):997–1002
- Ahmad I, Hussain T, Ashraf I, Nafees M, Rafay M, Iqbal M (2013a) Lethal effects of secondary metabolites in plant tissue culture. Am Eurasian J Agric Environ Sci 13(4):539–447
- Ahmad N, Faisal M, Anis M (2013b) Role of PGR on in vitro shoot propagation in Cyamopsis tetragonoloba L. (Taub.): a drought tolerant grain legume. Rend Lincei 24(1):7–12
- Arockiasamy S, Prakash S, Ignacimuthu S (2002) Direct organogenesis from mature leaf and petiole explants of Eryngium foetidum L. Biol Plant 45(1):129–132
- Babaei N, Abdullah NAP, Saleh G, Abdullah TL (2013) Control of contamination and explant browning in Curculigo latifolia in vitro cultures. J Med Plant Res 7(8):448–454
- Bidwell SD, Pederick JW, Sommer-Knudsen J, Woodrow IE (2001) Micropropagation of the nickel hyperaccumulator, Hybanthus floribundus (Family Violaceae). Plant Cell Tissue Organ Cult 67(1):89–92
- Blumenthal M, Goldberg A, Brinckmann J (2000) Herbal medicine: expanded commission E monographs. American Botanical Council, Newton, pp 233–236
- Chawla HS (2002) Introduction to plant biotechnology, 2nd rev edn. Science Publishers Inc., Enfield, pp 36–56
- Chen WH, Davey MR, Power JB, Cocking EC (1988) Control and maintenance of plant regeneration in sugarcane callus cultures. J Exp Bot 39:251–261
- CIMAP (1995) In vitro propagation of licorice through multiple shoot formation. Newsletter 21(4):4–5
- Crusheva R, Parvanov R (1978) Album of rare protected plants. Zemizdat Bulgaria, pp 27–28
- Dhandapani M, Kim DH, Hong SB (2008) Efficient plant regeneration via somatic embryogenesis and organogenesis from the explants of Catharanthus roseus. In Vitro Cell Dev Biol Plant 44(1):18–25
- Dodds JH, Roberts LW (1985) Experiments in plant tissue culture, 2nd edn. Cambridge Univ. Press, Cambridge, p 232
- Duke JA (1981) Handbook of legumes of world economic importance. Plenum Press, New York, pp 90–92
- Jain S, Kharya MD, Nayak S, Barik R (2008) Effect of antioxidants on callus browning of Glycyrrhiza glabra. J Nat Remedies 8(1):44–47
- Jaiswal N, Verma Y, Misra P (2017) Micropropagation and in vitro elicitation of licorice (Glycyrrhiza spp.). In Vitro Cell Dev Biol Plant 53:145–166
- Jan A, Bhat KM, Bhat SJA, Mir MA, Bhat MA, Imtiyaz AW, Rather JA (2013) Surface sterilization method for reducing microbial contamination of field grown strawberry explants intended for in vitro culture. Afr J Biotechnol 12:5749–5753
- Johnson M, Wesely EG, Kavitha MS, Uma V (2011) Antibacterial activity of leaves and inter-nodal callus extracts of Mentha arvensis L. Asian Pac J Trop Med 4(3):196–200
- Kakutani K, Ozaki K, Watanabe H, Tomoda K (1999) Somatic embryogenesis and plantlet regeneration on several species of licorice. Plant Biotechnol 16(3):239–241
- Kane M (2003) Bacterial and fungal indexing of tissue cultures. http://plant-tc.cfans.umn.edu/listserv/1996/log9612/indexing.htm. Accessed 3 Jan 2016
- Kikowska M, Thiem B, Sliwinska E, Rewers M, Kowalczyk M, Stochmal A, Oleszek W (2014) The effect of nutritional factors and plant growth regulators on micropropagation and production of phenolic acids and saponins from plantlets and adventitious root cultures of Eryngium maritimum L. J Plant Growth Regul 33(4):809–819
- Ko WH, Su CC, Chen CL, Chao CP (2009) Control of lethal browning of tissue culture plantlets of Cavendish banana cv. Formosana with ascorbic acid. Plant Cell Tissue Organ Cult 96(2):137–141
- Kojoma M, Ohyama K, Seki H, Hiraoka Y, Asazu SN, Sawa S, Sekizaki H, Yoshida S, Muranaka T (2010) In vitro proliferation and triterpenoid characteristics of licorice (Glycyrrhiza uralensis Fischer, Leguminosae) stolons. Plant Biotechnol 27(1):59–66
- Kovalenko P, Kurchii B (1998) Using of abscisic acid in the plant tissue culture of licorice Glycyrrhiza glabra L. electroporated protoplasts. II. In: International symposium on plant biotechnology, Kyiv, Ukraine, p 65
- Lalabadi MA, Jelodar NB, Bagheri N (2013) Effect of plant hormones on callus induction of explant types in endangered medicinal herb Tashnedari. Int J Biosci 3(12):39–43
- Lin GZ, Zhao XM, Hong SK, Lian YJ (2011) Somatic embryogenesis and shoot organogenesis in the medicinal plant Pulsatilla koreana Nakai. Plant Cell Tissue Organ Cult 106:93–103
- Liu B, Su S, Wu Y, Li Y, Shan X, Li S, Liu H, Dong H, Ding M, Han J, Yuan Y (2015) Histological and transcript analyses of intact somatic embryos in an elite maize (Zea mays L.) inbred line Y423. Plant Physiol Biochem 92:81–91
- Misaghi IJ, Donndelinger CR (1990) Endophytic bacteria in symptom- free cotton plants. Phytopathology 80:808–811
- Mousa NA, Siagura P, Wiryowidagdo S, Wagih ME (2007) Establishment of regenerative callus and cell suspension system of licorice (Glycyrrhiza glabra) for the production of the sweetener glycyrrhizin in vitro. Sugar Tech 9(1):72–82
- Murashige T, Skoog E (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Plant Physiol 15(3):473–497
- Murata M, Nishimura M, Murai N, Haruta M, Homma S, Itoh Y (2001) A transgenic apple callus showing reduced polyphenol oxidase activity and lower browning potential. Biosci Biotechnol Biochem 65(2):383–388
- Murthy HN, Hahn EJ, Paek KY (2008) Adventitious roots and secondary metabolism. Chin J Biotechnol 24(5):711–716
- Nassiri A, Hosseinzadeh H (2008) Review of pharmacological effects of Glycyrrhiza sp. and its biological compounds. Phytother Res 22:709–724
- Naz R, Anis M, Aref IM (2015) Management of cytokinin-auxin interactions for in vitro shoot proliferation of Althaeae officinalis L.: a valuable medicinal plant. Rend Lincei 26(3):323–334
- Ndakidemi CF, Ndakidemi PA (2013) The potential for developing an in vitro method for propagating Brachylaena huillensis (Silver oak). Am J Res Commun 1:224–241
- Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389
- Olukoga A, Donaldson D (1998) Historical perspective on health. The history of licorice: the plant, its extracts, cultivation, commercialisation and entomology. J R Soc Health 118(5):300–304
- Omamor IB, Asemota AO, Eke CR, Ezia EI (2007) Fungal contaminants of the oil palm tissue culture in Nigerian Institute for Oil Palm Research (NIFOR). Afr J Agric Res 2:534–537
- Poudyal BK, Du G, Zhang Y, Liu J, Shi Q (2008) Studies on browning problem and phenols content on shoots of Yali, Aikansui and Abbe Fetel pears for in vitro culture. Front Agric China 2(3):321–330
- Purohit SD, Dave A, Kukda G (1994) Micropropagation of safed musli (Chlorophytum borivilianum), a rare Indian medicinal herb. Plant Cell Tissue Organ Cult 39(1):93–96
- Rani G, Grover IS (1999) In vitro callus induction and regeneration studies in Withania somnifera. Plant Cell Tissue Organ Cult 57(1):23–27
- Rattanpal HS, Gill MIS, Sangwan AK (2011) Micropropagation of strawberry through meristem culture. Acta Hortic 890:149–154
- Sharma G, Nautiya AR (2009) Influence of explants type and plant growth regulators on in vitro multiple shoots regeneration of a Laurel from Himalaya. Nat Sci 7:1–7
- Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissue cultures in vitro. Symp Soc Exp Biol 11:118–131
- Slazak B, Sliwinska E, Saluga M, Ronikier M, Bujak J, Slomka A, Goransson U, Kuta E (2015) Micropropagation of Viola uliginosa (Violaceae) for endangered species conservation and for somaclonal variation-enhanced cyclotide biosynthesis. Plant Cell Tissue Organ Cult 120(1):179–190
- Thiem B, Kikowska M, Krawczyk A, Wieckowska B, Sliwinska E (2013) Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tissue Organ Cult 114(2):197–206
- Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26:618–631
- Tiwari S, Arya A, Kumar S (2012) Standardizing sterilization protocol and establishment of callus culture of sugarcane for enhanced plant regeneration in vitro. Res J Bot 7:1–7
- Verma P, Mathur AK (2011) Direct shoot bud organogenesis and plant regeneration from pre-plasmolysed leaf explants in Catharanthus roseus. Plant Cell Tissue Organ Cult 106(3):401–408
- Wani M, Pande S, More N (2010) Callus induction studies in Tridax procumbens L. Int J Biotechnol Appl 2(1):11–14
- Wojcik A, Podstolki A (2007) Leaf explants response in in vitro culture of St. John’s wort (Hypericum perforatum L.). Acta Physiol Plant 29(2):151–156
- Wongwicha W, Tanaka H, Shoyama Y, Tuvshintogtokh I, Putalun W (2008) Production of glycyrrhizin in callus cultures of Licorice. Z Naturforsch 63(5–6):413–417
Acknowledgements
This study was supported and financed by Sam Higginbottom Institute of Agriculture, Technology and Sciences, Allahabad, Uttar Pradesh, India.
Author Information
Department of Biochemistry and Biochemical Engineering, Jacob Institute of Biotechnology and Bioengineering, Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad, India
nancy.jaiswaal@gmail.com
Department of Biochemistry and Biochemical Engineering, Jacob Institute of Biotechnology and Bioengineering, Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad, India
Department of Molecular and Cellular Engineering, Jacob Institute of Biotechnology and Bioengineering, Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad, India